The potential role of extracellular regulatory kinase in the survival of patients with early stage adenocarcinoma
Introduction
Lung cancer is among the most common types of neoplasms and is the leading cause of death in the United States and the second major cause of death in Brazil (1-3). Unfortunately, the disease is usually advanced at the time of diagnosis: thus, precluding surgical treatment and restricting patients to chemotherapy and/or radiation therapy with a minimal chance of cure (4). Due to this aggressive behavior of lung cancer, it is necessary to identify molecules, proteins, or signaling pathways related to tumor growth, which have an influence on the outcome. The study of these prognostic factors could stimulate the development of new potential therapies targeting specific molecules (5).
A persistent search for prognostic biomarkers of nonsmall cell lung cancer (NSCLC) is currently ongoing (5). In this context, the epithelial growth factor receptor epidermal growth factor receptor (EGFR)-dependent RAS/RAF/MEK/ERK signaling cascade is under intensive investigation to identify new prognostic factors of lung cancer because they regulate signals for cell growth and survival and are involved in cell cycle regulation, angiogenesis, cell proliferation, and migration (6-8). A third of all forms of cancers are associated with enhanced activity of this cascade (9). For this reason, many EGFR (10,11), RAS, RAF, and MEK inhibitors have been developed as potential blockers of the activity or proliferation of different components of the extracellular signal-regulated kinase (ERK) signaling pathway (12-14). Therefore, the potential of overactive RAS, RAF, MEK, or ERK as prognostic factors for lung adenocarcinoma has become an interesting area of research.
Most of the studies searching for prognostic factors on NSCLC encompass all its types but do not discriminate in a specific cell type or samples from a particular stage, which makes it difficult to draw more detailed conclusions. Thus, it is essential to have a more specific population with minimal confusing factors to study potential prognostic factors in NSCLC. Based on these concerns, we selected only those patients who had been surgically treated for early lung adenocarcinoma to study the relationship of ERK and prognosis in this particular population.
We hypothesized that increased levels of activated ERK in patients with lung adenocarcinoma were associated with a poorer prognosis or decreased patient survival. Therefore, we conducted an immunohistochemical study using tumor samples from patients surgically treated for lung adenocarcinoma and performed a microarray analysis of experiments from different databases that included a population having a profile similar to that of our samples.
Materials and methods
We selected patients with lung adenocarcinoma who underwent pulmonary resection between 1998 and 2004 and were included in a previous study by Sánchez et al. (15). The present work was approved by the local Research Ethics Committee under protocol number 1852/08.
Surgical staging was determined according to the TNM classification system (16,17). All data related to preoperative evaluation, surgical techniques, and postoperative results are described in the article by Sánchez et al. (15). We randomly selected 36 patients with postoperative stage I or II disease and with available survival data and immunohistochemical samples.
Analysis of gene expression
Gene expression analyses were performed using microarray data from five different studies (18-22). However, we present data from a single study by Staaf et al., a database that included 39 adenocarcinoma patients with 72 available patients (GSE29016, Illumina HT-12 V3.0) (18). GSE29016 was retrieved from the gene expression database Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/), considering it was the only experiment that included all parameters analyzed in our study. To perform gene expression enrichment analysis, Gene Set Enrichment Analysis (GSEA) software, which requires a gene set, the parameters to be analyzed, and gene expression data for data processing was used. We used the Kyoto Encyclopedia of Genes and Genomes (KEGG) to obtain the gene set to be analyzed. Subsequently, we employed the online tool string to confirm interactions among the 261 genes of the group. The following parameters were analyzed: smoking status (8 nonsmokers and 21 smokers), TNM stage (30 with stage I and 9 with combined stage II + III disease, respectively), and sex (18 males and 21 females).
After the enrichment analysis was performed using GSEA as described by Mootha et al. (23), a survival curve for each of the parameters versus gene expression was plotted using GraphPad Prism 5.
Samples
Tissue samples for histopathological studies were obtained from surgical specimens of primary adenocarcinoma lesions from patients with surgical stage I or II. All samples were fixed in 10% buffered formalin and embedded in paraffin tissue blocks, which were then processed for immunohistochemical analyses. A tissue section was stained with hematoxylin and eosin (H&E) and analyzed by a pathologist to confirm the presence of adenocarcinoma in the sample.
Immunohistochemistry
Tissue sections measuring 4 cm in thickness were prepared on silanized slides. According to a previously standardized protocol, the following procedures were performed after deparaffinization and rehydration: heat-mediated antigen retrieval with sodium citrate buffer, blocking of endogenous peroxidase in a 5% H2O2 solution in methanol, and blocking of nonspecific binding in 1% bovine serum albumin (BSA) solution. Subsequently, the slides were incubated overnight at 4 °C with rabbit polyclonal antibody specific for the double phosphorylated form of ERK1/2 (Thr202/Tyr204; Cell Signaling, Beverly, MA, USA) diluted in a 1:300 ratio in 1% BSA. Plate washing, incubation, and color reaction were performed using the HRP-labeled conjugated polymer kit (Invitrogen®). Sections were then counterstained with hematoxylin. Negative controls were obtained using the same protocol, but without the primary antibody.
Analysis of immunohistochemical reactions
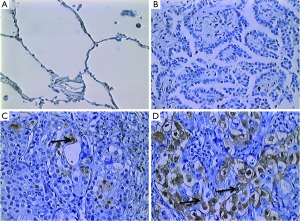
Immunohistochemical analyses were performed by counting ERK-positive cells/1,000 cells in consecutive microscopic fields, taking into account only tumor cells. Cell count was performed by two independent observers. A maximum interobserver discrepancy of 30% was considered or a third observer was included. Cell counting was performed using a Zeiss® Imager microscope coupled to the Image Pro Plus® 6.1 software. For statistical analysis, patients were divided into the following two groups based on a previous study: a group with <15% ERK-positive tumor cells and a group with ≥15% ERK-positive tumor cells (24) (Figure 1).
Statistical analyses
Mean values were compared using Student’s t-test, whereas categorical variables were compared using Fisher’s exact test. In addition, for survival analyses, we used Kaplan-Meier curves and Cox regression analyses in univariate and multivariate survival platforms. Statistical data were processed and analyzed using SPSS software, version 18.0 (IBM, USA, Chicago, 2009).
Results
Activated ERK levels were ≥15% and <15% in 21 (58%) and 15 (43%) patients, respectively (Figure 1). There were no statistically significant differences in age, sex, smoking history, and body mass index (BMI) between the groups stratified by ERK (Table 1). When survival was compared between the ERK ≥15% and ERK <15% groups, the former group showed lower survival than the latter during the study period (Table 2). We also performed statistical analyses using a cutoff threshold above and below 45% ERK positivity, and the results were very similar to those obtained when the cutoff threshold of above and below 15% were used.
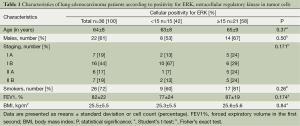
Full table
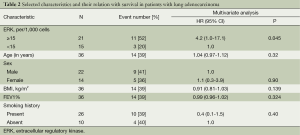
Full table
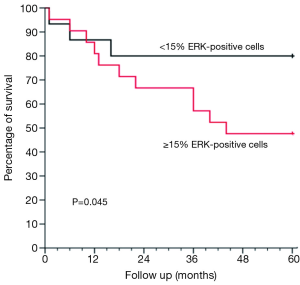
By using the multivariate Cox model after adjusting for age, sex, BMI, forced expiratory volume, and smoking history, the difference in survival between the ERK ≥15% and <15% groups was not only maintained but also statistically significant (P=0.045). This difference is shown graphically in Figure 2.
Enrichment analysis showed no correlation of variations in ERK gene expression in the 39 adenocarcinoma patients with stage I and combined stage II + III disease. Likewise, gender and smoking did not show a positive correlation with variations in ERK gene expression.
Discussion
Cancer is the result of the deregulation of multiple signaling pathways, and the inhibition of a single pathway may not be sufficient to trigger apoptosis or growth inhibition (6,25-27). ERK activation is involved in multiple cellular functions such as motility, proliferation, differentiation, and apoptosis (28,29). ERK activation influences the development and activation of normal cells as well as the abnormal proliferation of tumor cells (30,31). There is little evidence on the specific role of ERK activation in the development of NSCLC (24,32). By studying 111 patients with NSCLC, Vicent et al. demonstrated a positive correlation between ERK activation and later stages of the disease (24). Our study only examined patients with early stage adenocarcinoma who underwent surgical treatment: thus, constituting a population different from that described by Vicent et al. (24), which included patients with advanced stages and different histological types of lung carcinoma. Moreover, Vicent et al. separately analyzed ERK activation in the cytoplasm and nucleus and showed that there were no significant differences between these cell compartments (24). Therefore, we decided to collectively analyze these parameters in our study. However, Harding et al. suggested that receptor location, either in the plasma membrane or cytosol, may lead to different responses in the MAPK cascade activation (33). This finding is of great importance in the search for new therapies with ERK, and not MEK, as a target (34). It is believed that signal transduction is stronger in the plasma membrane and weaker in the cytosol (35). Therefore, activation of plasma membrane receptors would trigger a stronger cellular response, whereas ERK activation in the cytoplasm would affect only a subpopulation of cells, leading to an intermediate response (35). For this reason, future therapies would need to address differences in ERK cascade activation on the basis of receptor location. Furthermore, overexpression of this pathway may play an important role in the pathogenesis and progression of breast cancer and other cancers, making their components potential therapeutic targets (36-38).
We performed enrichment analysis using data from five different experiments (18-22), but because they yielded similar results, the dataset GSE29016 was selected because it was the only dataset containing all our study parameters. Our enrichment analysis had some limitations, in particular with regard to the number of patients diagnosed with adenocarcinoma. Only 39 patients were selected, and most of them were in stage I as verified in our dataset. Moreover, this experiment included few nonsmokers, which is expected in this disease group. Finally, our dataset contained more male patients, as opposed to the female predominance in the other datasets.
Our study demonstrated that high ERK levels are inversely correlated with survival. However, this was not revealed by the enrichment analysis, probably because of the sample type assayed (mRNA vs. protein) as well as the different patient profiles. In the literature, the presence of high ERK levels has been studied mainly in association with tumor aggressiveness and as potential therapeutic targets, and survival analyses have been restricted to only a few studies (39).
This study has several limitations that should be considered before interpreting the results. Our study included a small number of patients; however, they formed a specific group of individuals who did not show advanced stage disease and underwent surgical treatment. This also explains the limited number of patients with stage II disease. However, this makes the study population more homogeneous and helps in avoiding bias associated with the inclusion of more patients with an advanced stages disease, which tend to show higher ERK expression.
Conclusions
Taken together, our results suggest that higher ERK positivity in cells from biological samples of patients with lung adenocarcinoma patients is associated with tumor aggressiveness and poorer prognosis. Despite its limitations, this study demonstrates the importance of the role of ERK in lung adenocarcinomas and reinforces the need for additional studies to confirm our findings.
Acknowledgements
Authors’ contribution: S.L.M. conceived the study, collected the data, participated in the analysis of the samples and drafted the manuscript. C.B.M, R.T.M, M.C.F, R.M. collected the data, participated in the analysis of the samples and drafted the manuscript. M.B.S. participated in the microarray analysis. F.K, C.F.A. conceived the study, drafted and approved the manuscript final version.
This research was supported by grants from HCPA (Hospital de Clinicas de Porto Alegre Institutional Research Fund) and Universidade Federal do Rio Grande do Sul (UFRGS). We expressed our gratitude to Enago for proofreading this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Consonni D, De Matteis S, Lubin JH, et al. Lung cancer and occupation in a population-based case-control study. Am J Epidemiol 2010;171:323-33. [PubMed]
- Nana-Sinkam SP, Powell CA. Molecular biology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e30S-9S.
- Chanin TD, Merrick DT, Franklin WA, et al. Recent developments in biomarkers for the early detection of lung cancer: perspectives based on publications 2003 to present. Curr Opin Pulm Med 2004;10:242-7. [PubMed]
- Kreeger PK, Lauffenburger DA. Cancer systems biology: a network modeling perspective. Carcinogenesis 2010;31:2-8. [PubMed]
- Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 2005;6:827-37. [PubMed]
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene 2007;26:3113-21. [PubMed]
- Roskoski R Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res 2012;66:105-43. [PubMed]
- Ohashi K, Maruvka YE, Michor F, et al. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol 2013;31:1070-80. [PubMed]
- Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line--is there a difference? J Clin Oncol 2013;31:1081-8. [PubMed]
- Kulesza P, Ramchandran K, Patel JD. Emerging concepts in the pathology and molecular biology of advanced non-small cell lung cancer. Am J Clin Pathol 2011;136:228-38. [PubMed]
- Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat Rev Mol Cell Biol 2011;12:104-17. [PubMed]
- Balko JM, Jones BR, Coakley VL, et al. MEK and EGFR inhibition demonstrate synergistic activity in EGFR-dependent NSCLC. Cancer Biol Ther 2009;8:522-30. [PubMed]
- Sánchez PG, Vendrame GS, Madke GR, et al. Lobectomy for treating bronchial carcinoma: analysis of comorbidities and their impact on postoperative morbidity and mortality. J Bras Pneumol 2006;32:495-504. [PubMed]
- Rami-Porta R, Bolejack V, Goldstraw P. The new tumor, node, and metastasis staging system. Semin Respir Crit Care Med 2011;32:44-51. [PubMed]
- Pepek JM, Chino JP, Marks LB, et al. How well does the new lung cancer staging system predict for local/regional recurrence after surgery?: A comparison of the TNM 6 and 7 systems. J Thorac Oncol 2011;6:757-61. [PubMed]
- Staaf J, Jönsson G, Jönsson M, et al. Relation between smoking history and gene expression profiles in lung adenocarcinomas. BMC Med Genomics 2012;5:22. [PubMed]
- Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121-34. [PubMed]
- Landi MT, Dracheva T, Rotunno M, et al. Gene expression signature of cigarette smoking and its role in lung adenocarcinoma development and survival. PloS One 2008;3:e1651.
- Selamat SA, Chung BS, Girard L, et al. Genome-scale analysis of DNA methylation in lung adenocarcinoma and integration with mRNA expression. Genome Res 2012;22:1197-211. [PubMed]
- Takeuchi T, Tomida S, Yatabe Y, et al. Expression profile-defined classification of lung adenocarcinoma shows close relationship with underlying major genetic changes and clinicopathologic behaviors. J Clin Oncol 2006;24:1679-88. [PubMed]
- Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267-73. [PubMed]
- Vicent S, López-Picazo JM, Toledo G, et al. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br J Cancer 2004;90:1047-52. [PubMed]
- Ross JA, Rosen GD. The molecular biology of lung cancer. Curr Opin Pulm Med 2002;8:265-9. [PubMed]
- Jeanmart M, Lantuejoul S, Fievet F, et al. Value of immunohistochemical markers in preinvasive bronchial lesions in risk assessment of lung cancer. Clin Cancer Res 2003;9:2195-203. [PubMed]
- Greenberg AK, Lee MS. Biomarkers for lung cancer: clinical uses. Curr Opin Pulm Med 2007;13:249-55. [PubMed]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000;103:211-25. [PubMed]
- Cuevas BD, Abell AN, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene 2007;26:3159-71. [PubMed]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 2006;24:21-44. [PubMed]
- Dhanasekaran DN, Johnson GL. MAPKs: function, regulation, role in cancer and therapeutic targeting. Oncogene 2007;26:3097-9. [PubMed]
- Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalling pathways in cancer. Oncogene 2007;26:3279-90. [PubMed]
- Harding A, Tian T, Westbury E, et al. Subcellular localization determines MAP kinase signal output. Curr Biol 2005;15:869-73. [PubMed]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007;26:3291-310. [PubMed]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene 2007;26:3100-12. [PubMed]
- Sivaraman VS, Wang H, Nuovo GJ, et al. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest 1997;99:1478-83. [PubMed]
- Mandell JW, Hussaini IM, Zecevic M, et al. In situ visualization of intratumor growth factor signaling: immunohistochemical localization of activated ERK/MAP kinase in glial neoplasms. Am J Pathol 1998;153:1411-23. [PubMed]
- Adeyinka A, Nui Y, Cherlet T, et al. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res 2002;8:1747-53. [PubMed]
- Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res 2008;14:342-6. [PubMed]


