Ectopic mineralization in heart valves: new insights from in vivo and in vitro procalcific models and promising perspectives on noncalcifiable bioengineered valves
Introduction
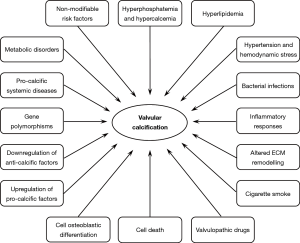
Anatomically located between the left ventricle outflow tract and the ascending aorta root, aortic valves play a key functional role in the cardiac cycle by enabling blood influx into the aorta lumen in response to ventricular systole and avoiding blood backflow into the left ventricle chamber during ventricular diastole. In such hemodynamics, valve cusps are subjected to recurring cycles of bending, shearing and tearing stresses, since they cyclically open and close about a billion times during a lifetime. It is thus not surprising that these specialized anatomic components frequently undergo structural alterations leading to two distinct congenital or acquired valvulopathies, i.e., aortic valve regurgitation and calcific aortic valve stenosis (CAVS). Aortic valve regurgitation is due to cusp weakening commonly associated with dilation of the ascending aorta root, causing improper valve closure with blood re-entry into the left ventricle chamber during ventricular diastole. Conversely, CAVS is characterized by cusp stiffness due to fibrosis and calcification, with obstruction of the left ventricular outflow tract leading to concentric left ventricle hypertrophy with associated angina, syncope and dyspnea (1). Valve calcification is a form of ectopic calcification due to very poor cusp vascularization with the consequent absence of an efficient macrophage-mediated scavenging of degenerated cells and their remnants, which act as early calcification foci. Valve calcification is conventionally distinguished into (I) metastatic calcification, i.e., diffused calcification due to systemic mineral imbalance, typically affecting uremic subjects, and (II) dystrophic calcification, i.e., topic calcification caused by tissue injury, aging and/or existence of other comorbidities, typically occurring in non-uremic subjects (2). Degenerative CAVS is the most common valve disorder caused by valve dystrophic calcification affecting the elderly population in the Western world (3). First described by Mönckeberg in 1904 (4), CAVS was regarded for years as a passive age-related process, with the major role being played by the prolonged shear-and-tear effect of hemodynamic stress on valve cusps (5-7). In the last two decades, the concept of CAVS etiopathogenesis has undergone considerable changes in that this valve disease is no longer regarded as the end stage of a mere degenerative process associated with cell death (8-11), but rather as a multifactorial calcific disorder including (I) accumulation of altered lipids, (II) release of proinflammatory mediators, (III) downregulated expression of anti-calcific factors, (IV) gene polymorphisms and (V) cell osteoblastic differentiation (12-22) (Figure 1). Concerning this latter aspect, reports have increased of possible transdifferentiation of aortic valve interstitial cells (AVICs) into osteoblast-like cells with heterotopic bone formation (23-28). Although these interesting results contribute to increasing knowledge on the processes underlying calcific valve disorders, they seem to have introduced the misconception that the terms “ectopic calcification” and “ectopic ossification” indicate the same pathological process. In this regard, it is worth pointing out that heterotopic bone formation was histologically shown to have occurred in less than 13% of thousands of explanted CAVS-affected valves (14,24) and actual osteocyte-like differentiation was observed in only one of 31 failed heart valves (23), strongly suggesting that valve ossification may be a mere epiphenomenon sometimes co-occurring with or superimposing on valve calcification, rather than this histogenetic process being regarded as a sine qua non for the occurrence of mineralizing processes. Ectopic calcification is also the main cause of failure in the mid to long term of bioprosthetic valves fabricated using native valves or pericardium harvested from animals (xenografts) or humans (allografts) (29,30). It was estimated that 20% to 30% of bioprosthetic valves require replacement within 10 years of implantation, with failed valve bioprostheses increasing up to 50%, or more in pediatric patients, within 15 years of implantation (2,29). Malfunctioning of implanted valve bioprostheses is reported to be due mainly to (I) cytotoxic effects exerted by treatments with glutaraldehyde (GA) or other crosslinking agents for xenografts (29,31-33), (II) tissue alterations caused by cryopreservation/thawing for allografts (29,34-37) and (III) occurrence of graft-versus-host rejection for both types (34,35,37-40). Disadvantages limiting the efficacy of the valve substitutes currently used in clinical practice in terms of biocompatibility, durability and capacity for tissue remodelling stimulated the development of novel tissue-engineered heart valves (TEHVs) (41). TEHVs can be obtained using synthetic or biological acellular scaffolds as well as scaffolds deriving from heart valves deprived of their resident cell population, all sharing the condition of being free of chemical treatments with any crosslinking agent. Such innovative bioengineered valves are being shown to permit both in vitro and in vivo cell repopulation, with good potential for tissue remodelling, adequate biocompatibility, proper mechanical behaviour and reduced propensity to mineralization (42-45), appearing as a promising alternative to the commercially available valve bioprostheses.
Ectopic calcification and cell death
Calcification is a widespread process that occurs physiologically in biological systems, from unicellular organisms to hard tissues belonging to Invertebrates and Vertebrates, besides involving soft tissues affected by various calcific diseases (46). In bones and teeth, major mineral nucleation sites are represented by cell-derived matrix vesicles, the production of which surely depends on distinct, active processes involving osteoblasts and odontoblasts, respectively (47-50). Release of analogous mineralizing matrix vesicles in association with apoptosis activation was also reported for hypertrophic chondrocytes in calcifying cartilages (51-53). In contrast, ectopic calcification in soft tissues is characterized by evident tissue alterations including different types of cell death, which have not yet been unequivocally identified. Despite a number of involved cell pathways having been highlighted, especially concerning apoptotic death, the major underlying problem is that a mare magnum exists of terms used to classify, more or less exhaustively, the various forms of cell death, with their mechanisms and pathogenetic factors still being far from a clear elucidation of their nature (54-56). It ensues that characterization of the upstream processes leading to the production of dying-cell-derived byproducts requires more knowledge to clearly distinguish how far and to what extent the so-called “matrix vesicles”, “apoptotic bodies”, “cell-derived bodies”, “membrane vesicle bodies”, “membrane blebs”, “exosomes” etc. are distinct structures or heteromorphic variants of the same form. In addition to these vesicular degenerative products, which are all cytoplasm-containing, membrane-bounded roundish bodies or polymorphic membranous debris derived from cells or cell organules, peculiar cell byproducts lined by alcianophilic thick walls were ultrastructurally identified in calcifying cardiovascular tissues (57,58). More recently, analogous peculiar structures named “PPL-vesicles” were ultrastructurally found to originate from the surface of pre-calcific dying AVICs after formation of a thick phthalocyanin-positive-layer (PPL) at their edges, as described below (59-65). Since it resulted that the sequential degenerative steps leading to PPL-vesicle release were quite different from those shown by cells undergoing the conventional cell death forms involved in valve calcification onset, i.e., apoptosis (8-10), necrosis (8) or autophagocytosis (11), it was suggested that an additional type of cell death might be associated with valve mineralization, as mentioned below. Further cell byproducts identified in a lot of calcified soft tissues, including native and bioprosthetic heart valves, consist of concentrically arranged, multi-laminated vesicular bodies that were named spherulites or calcospherulae (8,66-69). These structures appeared to act as early hydroxyapatite (HA) nucleators, with their electrondense layers representing an optimum substrate for the paracrystallin precipitation of radially oriented crystallites, which, in their turn, may promote additional autocatalytic mineral deposition involving wider and wider tissue areas. It is also of interest that synthetic vesicles ultrastructurally showing multilamellar envelopes made of lipid (70,71) or protein (72) anionic molecules were found to act as strong HA nucleators in in vitro environments.
Ectopic calcification, polyanions and cationic copper phthalocyanins
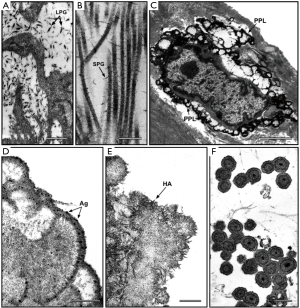
In the 1970s, lipid material accumulation was found to be strictly associated with increased precipitation of HA crystals in mineralizing hard and soft tissues in both physiological and pathological calcification processes. In particular, cell-membrane-derived acidic phospholipids forming the so-called “calcium-phospholipid-phosphate complexes” were originally identified in calcifying cartilage (73) and bone (74). Later, involvement of analogous anionic HA-nucleating complexes was found for pathological calcifications affecting soft tissues including vascular ones (75,76), in which additional identification of proteolipids suggested their contribution to ectopic mineralization (77,78). Moreover, Raman analysis of calcified human aortic valves and atherosclerotic plaques revealed carotenoids to be another lipid moiety that may play a role in pathological calcification of cardiovascular tissues (79-81). Consistently, lipid extraction from valve tissues before being subjected to experimental calcification was found to drastically reduce tissue mineralization (82,83). Similarly, lipid extraction from calcified valves was associated with complete removal of PPLs, revealing this lipid material to be the main substrate for HA crystal precipitation in aortic valves subjected to experimental calcification (59), as described below. Interestingly, lipid substances accumulating in calcified valve tissues were often found to undergo oxidation, so acquiring increased affinity for calcium ions (13,22,84). Consistently with their anionic nature, also ossification-related calcium-binding proteins, such as osteocalcin (16,23,85), osteopontin (16,23,86,87) and osteonectin (88), as well as proteoglycans rich in acidic glycosaminoglycan lateral chains (89-93) were reported to be involved in cardiovascular tissue calcification. In the past, copper phthalocyanins, such as alcian blue, cuprolinic blue (CB) and cupromeronic blue, proved to be the most effective histochemical reagents for the evidentiation of all types of polyanions, including extracellular glycosaminoglycans/proteoglycans (Figure 2A,B) and intracellular nucleic acids, in both light (94,95) and electron (96-98) microscopy. In early ultrastructural studies on calcified aortic valves and aorta walls, these cationic reagents revealed the presence of alcianophilic material at the surface of cells and cell debris, which was assumed to be formed by proteoglycans mixed with membrane-derived phospholipids (57,58). These fragmentary findings were later found to represent the latest intracellular stage of a multistep process, which was described after using phthalocyanins in ultrastructural studies on aortic valves or cultured AVICs subjected to experimental models of calcification (59-65). In particular, this procedural approach enabled an answer to be given to the following issues: (I) how the phthalocyanin-reactive acidic material is generated, (II) whether it actually represents a major HA nucleator and (III) what relationship exists between the phthalocyanin-reactive material and the extracellular structures actively or passively involved in calcification, such as collagen fibrils, elastin fibers and cell-derived calcospherulae. As a first step, the standard phthalocyanin-based staining procedure was modified by dissolving CB dye in an acidulated buffer (pH 4.8) to enable gentle unmasking of the phthalocyanin-positive material from superimposed HA crystallites concurrently with its in situ retention and staining. This modified histochemical technique was first applied on porcine aortic valves subjected to in vivo calcification induction using an animal model of xenogeneic valve subdermal implantation (59-62). In this context, peculiar PPLs were found to originate from colliquation of all cell membranous components, with intracellular release of an acidic amorphous material (PPM) and its subsequent centrifugal spreading and layering at the edges of dying AVICs (Figure 2C). Further release of PPL-vesicles due to blebbing of superficial PPLs was found to occur during later degenerative stages. Consistently with such a peculiar procalcific process, PPLs resulted as being formed mainly by acidic phospholipids, as revealed by different susceptibility to extraction and digestion procedures as well as positivity to suitably modified malachite-green-based histochemical techniques targeted at the identification of these lipids. Peripheral PPLs were found to play a major procalcific role, as revealed by massive clustering of HA crystals at the edges of cells and cell-derived debris in undecalcified samples as well as superimposed precipitation of silver particles onto surface PPLs in decalcified samples additionally subjected to a modified post-embedding von Kossa reaction suitably adapted for the ultrastructural evidencing of calcium-binding sites. Likewise, close association between PPL appearance and increases in calcium amounts estimated spectrophotometrically in parallel samples supported the evidence that a major procalcific role is played by PPLs during experimental valve calcification. The cell-derived PPL-vesicles observed were barely reminiscent of matrix vesicles because they resulted from cell blebbing, showed vesicular features and played an analogous procalcific role. However, they also appeared to be quite different from matrix vesicles, since they were edged by 120- to 600-nm-thick multilamellar PPLs instead of the orthodox 7-nm-thick bileaflet cell membrane and seemed not to contain cytoplasmic material. Moreover, a relationship was found to exist between such PPL-vesicles and interstitial calcospherulae, with the former clearly showing further rearrangement of the PPL substance into two to four irregularly spaced, concentric rings sometimes encircling an electrondense core. It is of note that extracellular matrix calcification was found to occur once collagen fibrils and elastin fibers were embedded by PPL material spreading from adjacent dead cells or PPL-vesicles, suggesting that valve mineralization is mainly primed by the procalcific degeneration of resident cells with just a secondary involvement of degenerating extracellular matrix components, as previously reported (99-101).
In vivo and in vitro experimental calcification of aortic valves
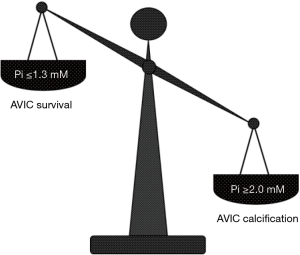
To shed light on the etiopathogenesis of heart valve calcification, procalcific animal models were developed, consisting of valve replacement in sheep and calves (102-105) or valve cusp subcutaneous implantation in rabbits or rats (104,106-108). Implanted valves and cusps were found to undergo mineralization showing histopathological features comparable to those of failed valve implants in humans, but with more accelerated kinetics (3 to 6 months for circulatory models and about 8 weeks for subcutaneous models), making such animal models also suitable for investigating the feasibility and effectiveness of potential anti-calcification strategies (109-111). In particular, using in vivo models of xenogeneic implantation of porcine aortic valve cusps into subdermal pouches surgically prepared in young rats, the main event in valve mineralization consisted of HA crystal precipitation at the level of roundish structures released by non-viable cells, which were initially described as matrix-vesicle-like bodies (99,104,107,112). Devitalization of valvular cells due to cusp fixation with 0.6% GA resulted as a prerequisite for valve mineralization occurrence, supporting the idea that the use of this chemical treatment for manufacturing of valve bioprostheses may be the most relevant cause of their failure (113). Consistently, non-fixed or alternatively processed valve cusps subjected to in vivo implantation were found to be free of mineralization (114-117). After using the rat subcutaneous implantation model, ultrastructural examination of explanted valve cusps subjected to pre-embedding CB-based histochemical reactions as described above revealed the presence of electrondense, lipid-rich PPLs layered at the periphery of degenerating cells (Figure 2C), which showed blebbing features and were involved in the formation of roundish cell-derived PPL-vesicles (59-61). Systematic ultrastructural studies on valve cusps explanted after increasing implantation times allowed the entire sequence of degenerative steps underlying the onset and progression of valve calcification to be defined (62), revealing the occurrence of a peculiar type of procalcific cell death characterized by a fast, dramatic breakdown of all cell membranous components, culminating with the detachment from dying AVICs of PPL-vesicles promoting subsequent calcification of the surrounding extracellular matrix, as described above. In the last two decades, several in vitro models were also developed to simulate etiopathogenetic environments promoting cell mineralization (118-122). Namely, smooth muscle cells or AVICs were cultured in media containing inorganic phosphate (Pi) at high concentrations (Pi ≥2.0 mM) and/or proinflammatory mediators that were expected to increase cell susceptibility to calcification. More recently, analogous procalcific in vitro models were set up, in which primary cultures of bovine AVICs were treated with different combinations of hyperphosphatemic- or normophosphatemic-like Pi concentrations, bacterial lipopolysaccharide (LPS) and conditioned medium obtained from cultures of LPS-stimulated macrophages (63-65). Despite identification of AVIC subtypes particularly prone to mineralization having been reported (122-124), entire AVIC populations were stimulated in these in vitro models in order to achieve a more faithful reproduction of native conditions. Ultrastructural analyses paralleled by spectrophotometric measurements of calcium amounts and alkaline phosphate activity supported the concept that cell exposure to high Pi concentrations is a prerequisite for priming AVIC mineralization, which was exacerbated by cell superstimulation with proinflammatory mediators (63,65). Using the CB-based histochemical procedures as above, also cultured AVICs appeared to undergo the same procalcific degeneration as that previously described for AVICs populating aortic valves subjected to in vivo calcification induction (Table 1), including accumulation of intracellular PPM, appearance of surface pro-calcific PPLs (Figure 2D and 2E), release of PPL-vesicles and their subsequent transformation into calcospherulae (Figure 2F). More information on cell response to non-calcific or sub-calcific environments was achieved by treating AVICs with different Pi concentrations like those spanning the normal range in organisms (65). Interestingly, two opposing Pi-dose-dependent cell responses were found to be evoked, i.e., cell survival versus procalcific cell death (Figure 3). Namely, AVICs exposed to low/medium Pi concentrations (0.8 and 1.3 mM) were found to undergo atypical autophagic activity in which a particularly hypertrophic endoplasmic reticulum appeared to be directly engaged in organelle sequestration and digestion, consistently with parallel time-dependent decreasing expression of autophagocytosis-related lysosomal markers. Since neither cells immunopositive for apoptosis markers nor cells showing degenerative features at ultrastructural level were encountered, the concept was supported that the observed non-lysosomal autophagic activity may correlate with AVIC survival. Conversely, AVICs treated with the highest Pi concentration (2.0 mM) were found to undergo mineralization following the same steps as those in the above-described lipid-release-associated procalcific cell death, supporting the concept that individuals with high normophosphatemic values are at increased risk of valve dystrophic mineralization. Since the in vitro models used allowed a reliable reproduction of AVIC calcification, including the genesis of calcospherulae as those observed in pathologically calcified heart valves (8,66,68), they could be usefully exploited on the one hand to find the molecular mechanisms associated with this type of cell death and on the other to assay the pro- or anti-calcific effects exerted on AVICs by putative stimulating or inhibitory agents, respectively.

Full table
Tissue-engineered heart valves
To date, surgical substitution of failed heart valves remains the leading therapeutic option for patients affected by valvular diseases (2,125). In the 1960s, mechanical valves were the first substitutes to be introduced in clinical practice, the Starr-Edwards caged-ball valve, Bjork-Shiley and Hall-Medtronic tilting-disk valves and St. Jude Medical bileaflet tilting-disk valve being the most widely used (29). Being prepared using synthetic materials such as titanium, cobalt-chromium alloy or pyrolytic carbon, mechanical implants have proven to be highly thrombogenic (126-133), requiring chronic anticoagulation therapies for transplanted patients and thus excluding their use in women of childbearing age (134-137). A further disadvantage is that implantation of mechanical valves in children requires reoperation in the medium term because these substitutes are unable to increase in size with the patient’s growth (138,139). Despite having shorter durability than mechanical devices, valve bioprostheses rapidly became widespread substitutes because of their native-like valve shape enabling better graft thromboresistance and adaptation to the hemodynamic flux (140-143). Bioprosthetic valves include (I) xenografts, which are usually fabricated using porcine valve cusps or bovine pericardium-derived limbs mounted (stent) or not (stentless) on a prosthetic metal frame and (II) allografts manufactured using human pericardium limbs or consisting of cryopreserved aortic valves harvested from human cadavers or “fresh” aortic valves excised from beating donor hearts at transplantation (29). Valve xenografts, such as the Hancock valve and Carpentier-Edwards valve, are prepared using animal tissues conventionally subjected to chemical crosslinking with GA, so allowing tissue sterilization and suppression of tissue immunogenicity due to retention of resident cells. Regrettably, such chemical fixation proved to be the major cause of xenograft failure because of GA-dependent cytotoxic effects and concurrent defective graft immunosuppression. Indeed, valve xenografts were found to be characterized by an absence of viable cells, besides showing calcific foci mainly at the level of the tunica spongiosa and cuspal commissures (31-33,107,144-146), with calcific deposits co-localizing with cell remnants, including interstitial calcospherulae, and collagen fibrils, as revealed ultrastructurally (66,68,69,147-152). Compared to xenografts, both cryopreserved and “fresh” valve allografts are expected to be better devices and, indeed, their slightly longer durability has been reported (153-159). However, these valve substitutes also showed some inconveniences such as (I) limited availability, (II) poor suitability for transplantation in pediatric patients (160-162) and, above all, (III) propensity to mineralization (35-37,163-168). Although no treatments with crosslinking agents are applied, sterilization procedures, cryopreservation-related damage and graft-versus-host rejection are suspected to contribute, alone or together, to allograft procalcific degeneration (34,169-174). Despite improvements being reported introducing alternative GA-free tissue treatments for xenografts (114-117,175-179) or less harmful cryopreservation and thawing procedures for allografts (180,181), tissue decellularization seems to be the most promising procedure to obtain biocompatible TEHVs free of adverse immune responses and calcification, being concurrently permissive of suitable cell repopulation, capable of tissue growth and remodelling and having adequate hemodynamic properties. In particular, detergent-based protocols utilizing SDS or sodium deoxycholate, also combined with Triton X-100, were found to be particularly suitable for tissue decellularization, providing acellular scaffolds with a well-preserved extracellular matrix texture and proper biomechanical behaviour, besides showing reduced susceptibility to tissue mineralization (182-187). Removal of resident cells with such decellularizing procedures offered the additional advantage of eliminating cell-associated antigens, which are responsible for the immunogenicity of valve bioprostheses including xenografts despite their chemical treatment with GA (188-190). Consistently, early failure of commercially available decellularized xenografts (SynerGraft, Matrix P) implanted in patients seemed to be ascribable to the use of defective cell removal procedures with persistence of cell remnants within valvular tissues (191,192). Conversely, clinical implantation of commercial decellularized-cryopreserved allografts (SynerGraft, CryoValve) gave very encouraging outcomes in terms of reduced antigenicity, retained structural integrity and long-term durability (193-199), although their clinical application is still hampered by their limited availability. First attempts to attain functional TEHVs were performed in vitro by statically seeding autologous or heterologous stem cells or differentiated cells on acellular synthetic or biological scaffolds as well as decellularized valve cusps. These constructs were found to undergo an almost complete re-endothelialization as well as side-by-side repopulation by seeded cells that acquired phenotypical features like those in native aortic valves (185,200-211). Subsequent use of bioreactors to test the resistance and hemodynamic behavior of repopulated scaffolds provided evidence that mechanical stresses stimulate the metabolic activity of entered cells with enhanced extracellular matrix remodelling (212-220). More recently, circulatory animal models have been preferred to dynamic bioreactors because they allow more reliable functional properties of implanted valve scaffolds to be obtained (221-226). Apart from non-human primates, pigs, rather than calves, dogs and the most often used sheep, have proved to be the animals that better replicate the anatomical and physiological features of human cardiovascular apparatus (227), including coagulation mechanisms and inflammatory system response (228). In particular, Vietnamese pigs appear to be an optimal choice to test the long-term follow up of implanted TEHVs because these miniature swine provide the additional advantages of (I) heart rate, cardiac stroke volume, mean arterial pressure and myocardial blood flow that almost coincide with those in humans (229) and (II) growth rates that are comparable to those of pediatric patients (230). Indeed, TEHVs implanted in mini-pigs were found to be suitably repopulated by native-like cells, with associated tissue growth and remodelling, besides showing good hemodynamic performances and limited tissue alterations even in the long-term follow up (231-234). The Vietnamese pig model was also used to test valve implants after cryopreservation and thawing, prefiguring a hypothetical scenario where cell removal from heart valves could become a standard procedure before their storage in valve cryobanks. Although these decellularized and cryopreserved porcine valves showed an acceptable functional activity, their structural features fell below expectations when histological and ultrastructural examinations revealed valve implants to be suitably covered by monolayered endothelium-like cells and populated by entered interstitial cells only in restricted cusp areas (235). These undesirable defects were reasonably ascribed to a sub-optimal preservation of graft extracellular matrix, with reduced propensity by host cells to enter, survive and properly differentiate. Once pre-implant valve scaffold cryopreservation procedures have been improved, cryobanks could be created of readily available, decellularized valve scaffolds capable of proper post-implant regeneration in order to meet the clinical demand for revitalizable pseudo-autologous valve substitutes.
Concluding remarks
Thanks to their morpho-functional features, heart valves enable a proper incessant cardiac cycle over an entire lifetime, withstanding the intermittent mechanical stresses due to hemodynamic pressure changes and blood flow friction. Accordingly, their malfunctions or breakdown relentlessly lead to life-threatening conditions. Of these anatomical elements, the aortic valves are the most commonly affected by calcification-associated disorders, with substantial improvements still being needed in predicting propensity to mineralization, providing effective drug therapies or designing surgical approaches to valve transplantation. The concept that calcific events affecting both native valves and bioprosthetic substitutes result from stress-dependent passive valve deterioration is being increasingly overtaken by an antithetical view that such a pathology depends on active processes involving valvular cells. Indeed, new findings are continually shedding light on possible mechanisms leading to valve mineralization even if its etiological triggers are still far from being exhaustively elucidated. Similarly, how much the calcific disease may be regarded as an intra-valve osteogenic process or rather a result of diffuse valve cell suffering compromising survival programs and/or leading to certain types of procalcific death has not yet been defined. Hence, additional investigation is required to shed light on inherent upstream regulatory mechanisms leading to ectopic mineralization. The existence of a specific sequence of procalcific intracellular and extracellular events underlying the calcific phenomenology was revealed ultrastructurally using adapted CB-based techniques in in vivo and in vitro models of valve calcification, as emphasized above, which may represent a trailblazer for better understanding this type of ectopic mineralization. Concerning surgical valve transplants, the most promising substitutes seem to be TEHVs because of their propensity to suitable cell repopulation, being free of undesirable calcific effects. In order to adopt personalized therapeutic approaches, proper pre-implant in vitro colonization of decellularized xenografts or, even better, allografts by seeded host-derived cells would be a time-consuming and complicated procedure. Instead, post-implant spontaneous in vivo cell repopulation of acellular scaffolds could be viewed as the best procedural choice also for their availability, once decellularized aortic valves can be methodically stored in cryobanks. Envisaging such a situation, preliminary investigation yielded results which, although not being entirely favourable, surely proved that decellularized and cryopreserved TEHVs do not oppose coating by endothelium-like cells and entering valve scaffolds by interstitial-like cells. In the light of such results, ongoing investigations providing concrete outcomes can be predicted with a certain optimism. In conclusion, although more than a few unresolved issues still persist, increased understanding on pathogenesis, onset and progression of valve calcification seems to be imminent as well as substantial improvements in the achievement of noncalcifiable pseudo-autologous TEHVs capable of the best functional performances in addition to longer durability and, possibly, post-operative growth.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hall RJ. Aortic valve disease. Eur Heart J 1984;5:135-9. [Crossref] [PubMed]
- Giachelli CM. Ectopic calcification. Am J Pathol 1999;154:671-5. [Crossref] [PubMed]
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Mönckeberg JG. Der normale histologische bau und die sklerose der aortenklappen. Virchows Arch Pathol Anat Physiol 1904;176:472-514. [Crossref]
- Clark RE, Finke EH. Scanning and light microscopy of human aortic leaflets in stressed and relaxed states. J Thorac Cardiovasc Surg 1974;67:792-804. [PubMed]
- Thubrikar MJ, Aouad J, Nolan SP. Patterns of calcific deposits in operatively excised stenotic or purely regurgitant aortic valves and their relation to mechanical stress. Am J Cardiol 1986;58:304-8. [Crossref] [PubMed]
- Beppu S, Suzuki S, Matsuda H, et al. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993;71:322-7. [Crossref] [PubMed]
- Kim KM. Apoptosis and calcification. Scanning Microsc 1995;9:1137-75; discussion 1175-8. [PubMed]
- Lee YS, Chou YY. Pathogenetic mechanism of senile calcific aortic stenosis: the role of apoptosis. Chin Med J (Engl) 1998;111:934-9. [PubMed]
- Jian B, Narula N, Li QY, et al. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg 2003;75:457-65; discussion 465-6. [Crossref] [PubMed]
- Somers P, Knaapen M, Kockx M, et al. Histological evaluation of autophagic cell death in calcified aortic valve stenosis. J Heart Valve Dis 2006;15:43-7; discussion 48. [PubMed]
- Otto CM, Kuusisto J, Reichenbach DD, et al. Characterization of the early lesion of “degenerative” valvular aortic stenosis. Circulation 1994;90:844-53. [Crossref] [PubMed]
- Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol 1999;19:1218-22. [Crossref] [PubMed]
- Mohler ER 3rd, Gannon F, Reynolds C, et al. Bone formation and inflammation in cardiac valves. Circulation 2001;103:1522-8. [Crossref] [PubMed]
- Kaden JJ, Dempfle CE, Grobholz R, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis 2003;170:205-11. [Crossref] [PubMed]
- Rajamannan NM, Subramaniam M, Rickard D, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 2003;107:2181-4. [Crossref] [PubMed]
- Ortlepp JR, Schmitz F, Mevissen V, et al. The amount of calcium-deficient hexagonal hydroxyapatite in aortic valves is influenced by gender and associated with genetic polymorphisms in patients with severe calcific aortic stenosis. Eur Heart J 2004;25:514-22. [Crossref] [PubMed]
- Liberman M, Bassi E, Martinatti MK, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008;28:463-70. [Crossref] [PubMed]
- Kestenbaum B, Glazer NL, Köttgen A, et al. Common genetic variants associate with serum phosphorus concentration. J Am Soc Nephrol 2010;21:1223-32. [Crossref] [PubMed]
- Lee JH, Meng X, Weyant MJ, et al. Stenotic aortic valves have dysfunctional mechanisms of anti-inflammation: implications for aortic stenosis. J Thorac Cardiovasc Surg 2011;141:481-6. [Crossref] [PubMed]
- Yutzey KE, Demer LL, Body SC, et al. Calcific aortic valve disease: a consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler Thromb Vasc Biol 2014;34:2387-93. [Crossref] [PubMed]
- Capoulade R, Chan KL, Yeang C, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol 2015;66:1236-46. [Crossref] [PubMed]
- Srivatsa SS, Harrity PJ, Maercklein PB, et al. Increased cellular expression of matrix proteins that regulate mineralization is associated with calcification of native human and porcine xenograft bioprosthetic heart valves. J Clin Invest 1997;99:996-1009. [Crossref] [PubMed]
- Steiner I, Kasparová P, Kohout A, et al. Bone formation in cardiac valves: a histopathological study of 128 cases. Virchows Arch 2007;450:653-7. [Crossref] [PubMed]
- Miller JD, Weiss RM, Serrano KM, et al. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arterioscler Thromb Vasc Biol 2010;30:2482-86. [Crossref] [PubMed]
- Boström KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res 2011;109:564-77. [Crossref] [PubMed]
- Wirrig EE, Hinton RB, Yutzey KE. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol 2011;50:561-9. [Crossref] [PubMed]
- Liu X, Xu Z. Osteogenesis in calcified aortic valve disease: from histopathological observation towards molecular understanding. Prog Biophys Mol Biol 2016;122:156-61. [Crossref] [PubMed]
- Schoen FJ, Levy RJ, Piehler HR. Pathological considerations in replacement cardiac valves. Cardiovasc Pathol 1992;1:29-52. [Crossref] [PubMed]
- Schoen FJ, Levy RJ. Pathology of substitute heart valves: new concepts and developments. J Card Surg 1994;9:222-7. [Crossref] [PubMed]
- Golomb G, Schoen FJ, Smith MS, et al. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprostheses. Am J Pathol 1987;127:122-30. [PubMed]
- Girardot MN, Torrianni M, Dillehay D, et al. Role of glutaraldehyde in calcification of porcine heart valves: comparing cusp and wall. J Biomed Mater Res 1995;29:793-801. [Crossref] [PubMed]
- Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology 2009;55:135-44. [Crossref] [PubMed]
- Neves J, Monteiro C, Santos R, et al. Histologic and genetic assessment of explanted allograft valves. Ann Thorac Surg 1995;60:S141-5. [Crossref] [PubMed]
- Schoen FJ, Mitchell RN, Jonas RA. Pathological considerations in cryopreserved allograft heart valves. J Heart Valve Dis 1995;4:S72-5; discussion S75-6.
- Brockbank KG, Lightfoot FG, Song YC, et al. Interstitial ice formation in cryopreserved homografts: a possible cause of tissue deterioration and calcification in vivo. J Heart Valve Dis 2000;9:200-6. [PubMed]
- Kitagawa T, Masuda Y, Tominaga T, et al. Cellular biology of cryopreserved allograft valves. J Med Invest 2001;48:123-32. [PubMed]
- Konakci KZ, Bohle B, Blumer R, et al. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest 2005;35:17-23. [Crossref] [PubMed]
- Park CS, Park SS, Choi SY, et al. Anti alpha-gal immune response following porcine bioprosthesis implantation in children. J Heart Valve Dis 2010;19:124-30. [PubMed]
- Park CS, Oh SS, Kim YE, et al. Anti-alpha-Gal antibody response following xenogeneic heart valve implantation in adults. J Heart Valve Dis 2013;22:222-9. [PubMed]
- Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920-6. [Crossref] [PubMed]
- Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng 2006;34:1799-819. [Crossref] [PubMed]
- Schoen FJ. Heart valve tissue engineering: quo vadis? Curr Opin Biotechnol 2011;22:698-705. [Crossref] [PubMed]
- Taylor DA, Sampaio LC, Gobin A. Building new hearts: a review of trends in cardiac tissue engineering. Am J Transplant 2014;14:2448-59. [Crossref] [PubMed]
- Kehl D, Weber B, Hoerstrup SP. Bioengineered living cardiac and venous valve replacements: current status and future prospects. Cardiovasc Pathol 2016;25:300-5. [Crossref] [PubMed]
- Bonucci E. Biological calcification: normal and pathological processes in the early stages. Berlin Heidelberg, Germany: Springer-Verlag; 2007.
- Schraer H, Gay CV. Matrix vesicles in newly synthesizing bone observed after ultracryotomy and ultramicroincineration. Calcif Tissue Res 1977;23:185-8. [Crossref] [PubMed]
- AlMuddaris MF, Dougherty WJ. The association of amorphous mineral deposits with the plasma membrane of pre- and young odontoblasts and their relationship to the origin of dentinal matrix vesicles in rat incisor teeth. Am J Anat 1979;155:223-44. [Crossref] [PubMed]
- Landis WJ, Paine MC, Hodgens KJ, et al. Matrix vesicles in embryonic chick bone: considerations of their identification, number, distribution, and possible effects on calcification of extracellular matrices. J Ultrastruct Mol Struct Res 1986;95:142-63. [Crossref] [PubMed]
- Kogaya Y, Furuhashi K. Ultrastructural localization of calcium in matrix vesicles and preodontoblasts of developing rat molar tooth germs during initial dentinogenesis. Acta Anat (Basel) 1988;132:100-8. [Crossref] [PubMed]
- Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res 1967;20:33-50. [Crossref] [PubMed]
- Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol 1969;41:59-72. [Crossref] [PubMed]
- Kirsch T, Swoboda B, Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage 2000;8:294-302. [Crossref] [PubMed]
- Narula J, Baliga R. What’s in a name? Would that which we call death by any other name be less tragic? Ann Thorac Surg 2001;72:1454-6. [Crossref] [PubMed]
- Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009;16:3-11. [Crossref] [PubMed]
- Vural F, Cebesoy S, Karakas M. Classification of cell death. J Entomol Zool Stud 2013;1:120-6.
- Kim KM, Huang S. Ultrastructural study of calcification of human aortic valve. Lab Invest 1971;25:357-66. [PubMed]
- Kim KM. Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc 1976;35:156-62. [PubMed]
- Ortolani F, Petrelli L, Tubaro F, et al. Novel ultrastructural features as revealed by phthalocyanine reactions indicate cell priming for calcification in subdermally implanted aortic valves. Connect Tissue Res 2002;43:44-55. [Crossref] [PubMed]
- Ortolani F, Tubaro F, Petrelli L, et al. Copper retention, calcium release and ultrastructural evidence indicate specific Cuprolinic Blue uptake and peculiar modifications in mineralizing aortic valves. Histochem J 2002;34:41-50. [Crossref] [PubMed]
- Ortolani F, Petrelli L, Nori SL, et al. Malachite green and phthalocyanine-silver reactions reveal acidic phospholipid involvement in calcification of porcine aortic valves in rat subdermal model. Histol Histopathol 2003;18:1131-40. [PubMed]
- Ortolani F, Bonetti A, Tubaro F, et al. Ultrastructural characterization of calcification onset and progression in subdermally implanted aortic valves. Histochemical and spectrometric data. Histol Histopathol 2007;22:261-72. [PubMed]
- Ortolani F, Rigonat L, Bonetti A, et al. Pro-calcific responses by aortic valve interstitial cells in a novel in vitro model simulating dystrophic calcification. Ital J Anat Embryol 2010;115:135-9. [PubMed]
- Bonetti A, Della Mora A, Contin M, et al. Ultrastructural and spectrophotometric study on the effects of putative triggers on aortic valve interstitial cells in in vitro models simulating metastatic calcification. Anat Rec (Hoboken) 2012;295:1117-27. [Crossref] [PubMed]
- Bonetti A, Della Mora A, Contin M, et al. Survival-related autophagic activity versus procalcific death in cultured aortic valve interstitial cells treated with critical normophosphatemic-like phosphate concentrations. J Histochem Cytochem 2017;65:125-38. [Crossref] [PubMed]
- Kim KM, Trump BF. Amorphous calcium precipitations in human aortic valve. Calcif Tissue Res 1975;18:155-60. [Crossref] [PubMed]
- Valente M, Bortolotti U, Thiene G. Ultrastructural substrates of dystrophic calcification in porcine bioprosthetic valve failure. Am J Pathol 1985;119:12-21. [PubMed]
- Lee YS. Morphogenesis of calcification in porcine bioprostheses: insights from high resolution electron microscopic investigation at molecular and atomic resolution. J Electron Microsc (Tokyo) 1993;42:156-65. [PubMed]
- Bottio T, Thiene G, Pettenazzo E, et al. Hancock II bioprosthesis: a glance at the microscope in mid-long-term explants. J Thorac Cardiovasc Surg 2003;126:99-105. [Crossref] [PubMed]
- Heywood BR, Eanes ED. An ultrastructural study of calcium phosphate formation in multilamellar liposome suspensions. Calcif Tissue Int 1987;41:192-201. [Crossref] [PubMed]
- Eanes ED. Biophysical aspects of lipid interaction with mineral: liposome model studies. Anat Rec 1989;224:220-5. [Crossref] [PubMed]
- Amos FF, Dai L, Kumar R, et al. Mechanism of formation of concentrically laminated spherules: implication to Randall’s plaque and stone formation. Urol Res 2009;37:11-7. [Crossref] [PubMed]
- Wuthier RE. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochem Biophys Acta 1975;409:128-43. [Crossref] [PubMed]
- Boskey AL, Posner AS. Extraction of a calcium-phospholipid-phosphate complex from bone. Calcif Tissue Res 1976;19:273-83. [Crossref] [PubMed]
- Dmitrovsky E, Boskey AL. Calcium-acidic phospholipid-phosphate complexes in human atherosclerotic aortas. Calcif Tissue Int 1985;37:121-5. [Crossref] [PubMed]
- Boskey AL, Bullough PG, Vigorita V, et al. Calcium-acidic phospholipid-phosphate complexes in human hydroxyapatite-containing pathologic deposits. Am J Pathol 1988;133:22-9. [PubMed]
- Ennever J, Vogel JJ, Riggan LJ. Calcification by proteolipid from atherosclerotic aorta. Atherosclerosis 1980;35:209-13. [Crossref] [PubMed]
- Romeo R, Augustyn JM, Mandel G. Isolation and characterization of human apatite-inducing aortic proteolipid. Exp Mol Pathol 1989;51:149-58. [Crossref] [PubMed]
- Römer TJ, Brennan JF 3rd, Fitzmaurice M, et al. Histopathology of human coronary atherosclerosis by quantifying its chemical composition with Raman spectroscopy. Circulation 1998;97:878-85. [Crossref] [PubMed]
- van de Poll SW, Kastelijn K, Bakker Schut TC, et al. On-line detection of cholesterol and calcification by catheter based Raman spectroscopy in human atherosclerotic plaque ex vivo. Heart 2003;89:1078-82. [Crossref] [PubMed]
- Bonetti A, Bonifacio A, Della Mora A, et al. Carotenoids co-localize with hydroxyapatite, cholesterol, and other lipids in calcified stenotic aortic valves. Ex vivo Raman maps compared to histological patterns. Eur J Histochem 2015;59:2505. [Crossref] [PubMed]
- Jorge-Herrero E, Fernández P, de la Torre N, et al. Inhibition of the calcification of porcine valve tissue by selective lipid removal. Biomaterials 1994;15:815-20. [Crossref] [PubMed]
- Nimni ME, Myers D, Ertl D, et al. Factors which affect the calcification of tissue-derived bioprostheses. J Biomed Mater Res 1997;35:531-7. [Crossref] [PubMed]
- Lehti S, Käkelä R, Hörkkö S, et al. Modified lipoprotein-derived lipid particles accumulate in human stenotic aortic valves. PLoS One 2013;8:e65810. [Crossref] [PubMed]
- Levy RJ, Gundberg C, Scheinman R. The identification of the vitamin K-dependent bone protein osteocalcin as one of the gamma-carboxyglutamic acid containing proteins present in calcified atherosclerotic plaque and mineralized heart valves. Atherosclerosis 1983;46:49-56. [Crossref] [PubMed]
- Fitzpatrick LA, Severson A, Edwards WD, et al. Diffuse calcification in human coronary arteries. Association of osteopontin with atherosclerosis. J Clin Invest 1994;94:1597-604. [Crossref] [PubMed]
- O'Brien KD, Kuusisto J, Reichenbach DD, et al. Osteopontin is expressed in human aortic valvular lesions. Circulation 1995;92:2163-8. [Crossref] [PubMed]
- Charest A, Pépin A, Shetty R, et al. Distribution of SPARC during neovascularisation of degenerative aortic stenosis. Heart 2006;92:1844-9. [Crossref] [PubMed]
- Völker W, Schmidt A, Buddecke E. Cytochemical changes in a human arterial proteoglycan related to atherosclerosis. Atherosclerosis 1989;77:117-30. [Crossref] [PubMed]
- Jorge-Herrero E, Fernández P, Gutiérrez M, et al. Study of the calcification of bovine pericardium: analysis of the implication of lipids and proteoglycans. Biomaterials 1991;12:683-9. [Crossref] [PubMed]
- Shepard N. Role of proteoglycans in calcification. In: Bonucci E, editor. Calcification in biological systems. Boca Raton, FL: CRC Press, 1992:41-58.
- Vyavahare N, Ogle M, Schoen FJ, et al. Mechanisms of bioprosthetic heart valve failure: fatigue causes collagen denaturation and glycosaminoglycan loss. J Biomed Mater Res 1999;46:44-50. [Crossref] [PubMed]
- Simionescu DT, Lovekamp JJ, Vyavahare NR. Glycosaminoglycan-degrading enzymes in porcine aortic heart valves: implications for bioprosthetic heart valve degeneration. J Heart Valve Dis 2003;12:217-25. [PubMed]
- Scott JE, Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie 1965;5:221-33. [Crossref] [PubMed]
- Scott JE. Histochemistry of Alcian Blue: III. The molecular biological basis of staining by Alcian Blue 8GX and analogous phthalocyanins. Histochemie 1972;32:191-212. [Crossref] [PubMed]
- Ruggeri A, Dell’Orbo C, Quacci D. Electron microscopic visualization of proteoglycans with Alcian Blue. Histochem J 1975;7:187-97. [Crossref] [PubMed]
- Scott JE. Localization of proteoglycans in tendon by electron microscopy. Biochem J 1980;187:887-91. [Crossref] [PubMed]
- Reale E, Luciano L, Spitznas M. Histochemical demonstration of hyaluronic acid molecules by alcian blue. Histochem J 1986;18:306-16. [Crossref] [PubMed]
- Schoen FJ, Levy RJ. Bioprosthetic heart valve calcification: membrane-mediated events and alkaline phosphatase. Bone Miner 1992;17:129-33. [Crossref] [PubMed]
- Kirsch T, Harrison G, Golub EE, et al. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem 2000;275:35577-83. [Crossref] [PubMed]
- Kim KM. Cells, rather than extracellular matrix, nucleate apatite in glutaraldehyde-treated vascular tissue. J Biomed Mater Res 2002;59:639-45. [Crossref] [PubMed]
- Barnhart GR, Jones M, Ishihara T, et al. Failure of porcine aortic and bovine pericardial prosthetic valves: an experimental investigation in young sheep. Circulation 1982;66:I150-3. [PubMed]
- Levy RJ, Zenker JA, Bernhard WF. Porcine bioprosthetic valve calcification in bovine left ventricle-aorta shunts: studies of the deposition of vitamin K-dependent proteins. Ann Thorac Surg 1983;36:187-92. [Crossref] [PubMed]
- Schoen FJ, Levy RJ, Nelson AC, et al. Onset and progression of experimental bioprosthetic heart valve calcification. Lab Invest 1985;52:523-32. [PubMed]
- Schoen FJ, Hirsch D, Bianco RW, et al. Onset and progression of calcification in porcine aortic bioprosthetic valves implanted as orthotopic mitral valve replacements in juvenile sheep. J Thorac Cardiovasc Surg 1994;108:880-7. [PubMed]
- Fishbein MC, Levy RJ, Ferrans VJ, et al. Calcifications of cardiac valve bioprostheses. Biochemical, histologic, and ultrastructural observations in a subcutaneous implantation model system. J Thorac Cardiovasc Surg 1982;83:602-9. [PubMed]
- Levy RJ, Schoen FJ, Levy JT, et al. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde-preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am J Pathol 1983;113:143-55. [PubMed]
- Mako WJ, Vesely I. In vivo and in vitro models of calcification in porcine aortic valve cusps. J Heart Valve Dis 1997;6:316-23. [PubMed]
- Levy RJ, Wolfrum J, Schoen FJ, et al. Inhibition of calcification of bioprosthetic heart valves by local controlled-release diphosphonate. Science 1985;228:190-2. [Crossref] [PubMed]
- Jones M, Eidbo EE, Hilbert SL, et al. Anticalcification treatments of bioprosthetic heart valves: in vivo studies in sheep. J Card Surg 1989;4:69-73. [Crossref] [PubMed]
- Webb CL, Schoen FJ, Flowers WE, et al. Inhibition of mineralization of glutaraldehyde-pretreated bovine pericardium by AlCl3. Mechanisms and comparisons with FeCl3, LaCl3, and Ga(NO3)3 in rat subdermal model studies. Am J Pathol 1991;138:971-81. [PubMed]
- Maxwell L, Gavin JB, Barratt-Boyes BG. Differences between heart valve allografts and xenografts in the incidence and initiation of dystrophic calcification. Pathology 1989;21:5-10. [Crossref] [PubMed]
- Gong G, Ling Z, Seifter E, et al. Aldehyde tanning: the villain in bioprosthetic calcification. Eur J Cardiothorac Surg 1991;5:288-99. [Crossref] [PubMed]
- Girardot JM, Girardot MN. Amide cross-linking: an alternative to glutaraldehyde fixation. J Heart Valve Dis 1996;5:518-25. [PubMed]
- Moore MA, McIlroy BK, Phillips RE Jr. Nonaldehyde sterilization of biologic tissue for use in implantable medical devices. ASAIO J 1997;43:23-30. [Crossref] [PubMed]
- Moore MA, Adams AK. Calcification resistance, biostability, and low immunogenic potential of porcine heart valves modified by dye-mediated photooxidation. J Biomed Mater Res 2001;56:24-30. [Crossref] [PubMed]
- Connolly JM, Alferiev I, Clark-Gruel JN, et al. Triglycidylamine crosslinking of porcine aortic valve cusps or bovine pericardium results in improved biocompatibility, biomechanics, and calcification resistance: chemical and biological mechanisms. Am J Pathol 2005;166:1-13. [Crossref] [PubMed]
- Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 2000;87:E10-7. [Crossref] [PubMed]
- Tintut Y, Patel J, Territo M, et al. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 2002;105:650-5. [Crossref] [PubMed]
- Mathieu P, Voisine P, Pépin A, et al. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis 2005;14:353-7. [PubMed]
- Babu AN, Meng X, Zou N, et al. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg 2008;86:71-6. [Crossref] [PubMed]
- Rattazzi M, Iop L, Faggin E, et al. Clones of interstitial cells from bovine aortic valve exhibit different calcifying potential when exposed to endotoxin and phosphate. Arterioscler Thromb Vasc Biol 2008;28:2165-72. [Crossref] [PubMed]
- Mohler ER 3rd, Chawla MK, Chang AW, et al. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis 1999;8:254-60. [PubMed]
- Masjedi S, Amarnath A, Baily KM, et al. Comparison of calcification potential of valvular interstitial cells isolated from individual aortic valve cusps. Cardiovasc Pathol 2016;25:185-94. [Crossref] [PubMed]
- Schoen FJ, Gotlieb AI. Heart valve health, disease, replacement, and repair: a 25-year cardiovascular pathology perspective. Cardiovasc Pathol 2016;25:341-52. [Crossref] [PubMed]
- Björk VO, Henze A. Ten years' experience with the Björk-Shiley tilting disc valve. J Thorac Cardiovasc Surg 1979;78:331-42. [PubMed]
- Baudet EM, Oca CC, Roques XF, et al. A 5 1/2 year experience with the St. Jude Medical cardiac valve prosthesis. Early and late results of 737 valve replacements in 671 patients. J Thorac Cardiovasc Surg 1985;90:137-44. [PubMed]
- Yamak B, Karagöz HY, Zorlutuna Y, et al. Low-dose anticoagulant management of patients with St. Jude Medical mechanical valve prostheses. Thorac Cardiovasc Surg 1993;41:38-42. [Crossref] [PubMed]
- Akins CW. Long-term results with the Medtronic-Hall valvular prosthesis. Ann Thorac Surg 1996;61:806-13. [Crossref] [PubMed]
- Kayali MT, Fetieh MW, Abdulsalam MA, et al. Thrombotic obstruction of bileaflet mechanical prosthetic heart valves: early diagnosis and management. J Cardiovasc Surg (Torino) 1998;39:331-5. [PubMed]
- Lund O, Nielsen SL, Arildsen H, et al. Standard aortic St. Jude valve at 18 years: performance profile and determinants of outcome. Ann Thorac Surg 2000;69:1459-65. [Crossref] [PubMed]
- Dürrleman N, Pellerin M, Bouchard D, et al. Prosthetic valve thrombosis: twenty-year experience at the Montreal Heart Institute. J Thorac Cardiovasc Surg 2004;127:1388-92. [Crossref] [PubMed]
- Hart EA, Jansen R, Meijs TA, et al. Anticoagulant bridging in left-sided mechanical heart valve patients. Int J Cardiol 2017;232:121-6. [Crossref] [PubMed]
- Larrea JL, Núñez L, Reque JA, et al. Pregnancy and mechanical valve prostheses: a high-risk solution for the mother and the fetus. Ann Thorac Surg 1983;36:459-63. [Crossref] [PubMed]
- Born D, Martinez EE, Almeida PA, et al. Pregnancy in patients with prosthetic heart valves: the effects of anticoagulation on mother, fetus, and neonate. Am Heart J 1992;124:413-7. [Crossref] [PubMed]
- Vitale N, De Feo M, De Santo LS, et al. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol 1999;33:1637-41. [Crossref] [PubMed]
- Al-Lawati AA, Venkitraman M, Al-Delaime T, et al. Pregnancy and mechanical heart valves replacement; dilemma of anticoagulation. Eur J Cardiothorac Surg 2002;22:223-7. [Crossref] [PubMed]
- Lupinetti FM, Warner J, Jones TK, et al. Comparison of human tissues and mechanical prostheses for aortic valve replacement in children. Circulation 1997;96:321-5. [Crossref] [PubMed]
- Larsen SH, Houlind K, Hansen OK, et al. Medium-term follow-up of mechanical valves inserted in children. Cardiol Young 2006;16:579-85. [Crossref] [PubMed]
- Farah E, Enriquez-Sarano M, Vahanian A, et al. Thromboembolic and haemorrhagic risk in mechanical and biological aortic prostheses. Eur Heart J 1984;5:43-7. [Crossref] [PubMed]
- Martinell J, Fraile J, Artiz V, et al. Long-term comparative analysis of the Björk-Shiley and Hancock valves implanted in 1975. J Thorac Cardiovasc Surg 1985;90:741-9. [PubMed]
- Schoen FJ, Hobson CE. Anatomic analysis of removed prosthetic heart valves: causes of failure of 33 mechanical valves and 58 bioprostheses, 1980 to 1983. Hum Pathol 1985;16:549-59. [Crossref] [PubMed]
- Borkon AM, Soule LM, Baughman KL, et al. Comparative analysis of mechanical and bioprosthetic valves after aortic valve replacement. J Thorac Cardiovasc Surg 1987;94:20-33. [PubMed]
- Schoen FJ, Tsao JW, Levy RJ. Calcification of bovine pericardium used in cardiac valve bioprostheses. Implications for the mechanisms of bioprosthetic tissue mineralization. Am J Pathol 1986;123:134-45. [PubMed]
- Maranto AR, Schoen FJ. Alkaline phosphatase activity of glutaraldehyde-treated bovine pericardium used in bioprosthetic cardiac valves. Circ Res 1988;63:844-8. [Crossref] [PubMed]
- Simionescu DT, Lovekamp JJ, Vyavahare NR. Extracellular matrix degrading enzymes are active in porcine stentless aortic bioprostheses heart valves. J Biomed Mater Res A 2003;66:755-63. [Crossref] [PubMed]
- Fishbein MC, Gissen SA, Collins JJ Jr, et al. Pathologic findings after cardiac valve replacement with glutaraldehyde-fixed porcine valves. Am J Cardiol 1977;40:331-7. [Crossref] [PubMed]
- Spray TL, Roberts WC. Structural changes in porcine xenografts used as substitute cardiac valves. Gross and histologic observations in 51 glutaraldehyde-preserved Hancock valves in 41 patients. Am J Cardiol 1977;40:319-30. [Crossref] [PubMed]
- Ferrans VJ, Boyce SW, Billingham ME, et al. Calcific deposits in porcine bioprostheses: structure and pathogenesis. Am J Cardiol 1980;46:721-34. [Crossref] [PubMed]
- Stein PD, Kemp SR, Riddle JM, et al. Relation of calcification to torn leaflets of spontaneously degenerated porcine bioprosthetic valves. Ann Thorac Surg 1985;40:175-80. [Crossref] [PubMed]
- Valente M, Minarini M, Thiene G, et al. The pathology of Hancock standard porcine valve prosthesis: a 20-year span of experience. J Card Surg 1990;5:328-35. [Crossref] [PubMed]
- Biedrzycki LM, Lerner E, Levy RJ, et al. Differential calcification of cusps and aortic wall of failed stented porcine bioprosthetic valves. J Biomed Mater Res 1997;34:411-5. [Crossref] [PubMed]
- Matsuki O, Robles A, Gibbs S, et al. Long-term performance of 555 aortic homografts in the aortic position. Ann Thorac Surg 1988;46:187-91. [Crossref] [PubMed]
- Angell WW, Oury JH, Lamberti JJ, et al. Durability of the viable aortic allograft. J Thorac Cardiovasc Surg 1989;98:48-55; discussion 55-6. [PubMed]
- Shapira OM, Shemin RJ. Aortic valve replacement with cryopreserved allografts: mid-term results. J Card Surg 1994;9:292-7. [Crossref] [PubMed]
- Yacoub M, Rasmi NR, Sundt TM, et al. Fourteen-year experience with homovital homografts for aortic valve replacement. J Thorac Cardiovasc Surg 1995;110:186-93; discussion 193-4. [Crossref] [PubMed]
- D’Alfonso A, Verunelli F, Mariani MA, et al. Aortic homograft improves hemodynamic performance and clinical outcome at mid-term follow-up. Ital Heart J 2004;5:453-9. [PubMed]
- Kaya A, Schepens MA, Morshuis WJ, et al. Valve-related events after aortic root replacement with cryopreserved aortic homografts. Ann Thorac Surg 2005;79:1491-5. [Crossref] [PubMed]
- Kilian E, Fries F, Kowert A, et al. Homograft implantation for aortic valve replacement since 15 years: results and follow-up. Heart Surg Forum 2010;13:E238-42. [Crossref] [PubMed]
- Clarke DR, Campbell DN, Hayward AR, et al. Degeneration of aortic valve allografts in young recipients. J Thorac Cardiovasc Surg 1993;105:934-41; discussion 941-2. [PubMed]
- Yankah AC, Alexi-Meskhishvili V, Weng Y, et al. Accelerated degeneration of allografts in the first two years of life. Ann Thorac Surg 1995;60:S71-7. [Crossref] [PubMed]
- Rajani B, Mee RB, Ratliff NB. Evidence of rejection of homograft cardiac valves in infants. J Thorac Cardiovasc Surg 1998;115:111-7. [Crossref] [PubMed]
- Moore CH, Martelli V, Al-Janabi N, et al. Analysis of homograft valve failure in 311 patients followed up to 10 years. Ann Thorac Surg 1975;20:274-81. [Crossref] [PubMed]
- Lorch G, Kennedy JW, Gould KL. Severe stenosis of a viable homograft aortic valve. J Thorac Cardiovasc Surg 1976;71:932-3. [PubMed]
- Lis GJ, Rokita E, Podolec P, et al. Mineralization and organic phase modifications as contributory factors of accelerated degeneration in homograft aortic valves. J Heart Valve Dis 2003;12:741-51. [PubMed]
- Podolec P, Drwila R, Goncerz G, et al. Fresh-wet storage accelerates aortic homograft calcification. Cell Tissue Bank 2008;9:37-40. [Crossref] [PubMed]
- Ravenni G, Pratali S, Scioti G, et al. Total calcification of an aortic homograft used as aortic root replacement. J Cardiovasc Med (Hagerstown) 2011;12:191-2. [Crossref] [PubMed]
- Nappi F, Al-Attar N, Spadaccio C, et al. Aortic valve homograft: 10-year experience. Surg Technol Int 2014;24:265-72. [PubMed]
- Yankah AC, Wottge HU, Muller-Hermelink HK, et al. Transplantation of aortic and pulmonary allografts, enhanced viability of endothelial cells by cryopreservation, importance of histocompatibility. J Card Surg 1987;2:209-20. [Crossref] [PubMed]
- Hoekstra F, Knoop C, Vaessen L, et al. Donor-specific cellular immune response against human cardiac valve allografts. J Thorac Cardiovasc Surg 1996;112:281-6. [Crossref] [PubMed]
- Hogan P, Duplock L, Green M, et al. Human aortic valve allografts elicit a donor-specific immune response. J Thorac Cardiovasc Surg 1996;112:1260-6; discussion 1266-7. [Crossref] [PubMed]
- Hawkins JA, Breinholt JP, Lambert LM, et al. Class I and class II anti-HLA antibodies after implantation of cryopreserved allograft material in pediatric patients. J Thorac Cardiovasc Surg 2000;119:324-30. [Crossref] [PubMed]
- Welters MJ, Oei FB, Witvliet MD, et al. A broad and strong humoral immune response to donor HLA after implantation of cryopreserved human heart valve allografts. Hum Immunol 2002;63:1019-25. [Crossref] [PubMed]
- Pompilio G, Polvani G, Piccolo G, et al. Six-year monitoring of the donor-specific immune response to cryopreserved aortic allograft valves: implications with valve dysfunction. Ann Thorac Surg 2004;78:557-63. [Crossref] [PubMed]
- Weissenstein C, Human P, Bezuidenhout D, et al. Glutaraldehyde detoxification in addition to enhanced amine cross-linking dramatically reduces bioprosthetic tissue calcification in the rat model. J Heart Valve Dis 2000;9:230-40. [PubMed]
- Lovekamp J, Vyavahare N. Periodate-mediated glycosaminoglycan stabilization in bioprosthetic heart valves. J Biomed Mater Res 2001;56:478-86. [Crossref] [PubMed]
- Aimoli CG, Nogueira GM, Nascimento LS, et al. Lyophilized bovine pericardium treated with a phenethylamine-diepoxide as an alternative to preventing calcification of cardiovascular bioprosthesis: preliminary calcification results. Artif Organs 2007;31:278-83. [Crossref] [PubMed]
- Somers P, De Somer F, Cornelissen M, et al. Genipin blues: an alternative non-toxic crosslinker for heart valves? J Heart Valve Dis 2008;17:682-8. [PubMed]
- Figueiredo RL, Dantas MS, Oréfice RL. Thermal welding of biological tissues derived from porcine aorta for manufacturing bioprosthetic cardiac valves. Biotechnol Lett 2011;33:1699-703. [Crossref] [PubMed]
- Brockbank KG, Schenke-Layland K, Greene ED, et al. Ice-free cryopreservation of heart valve allografts: better extracellular matrix preservation in vivo and preclinical results. Cell Tissue Bank 2012;13:663-71. [Crossref] [PubMed]
- Manuchehrabadi N, Gao Z, Zhang J, et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci Transl Med 2017;9. [Crossref] [PubMed]
- Booth C, Korossis SA, Wilcox HE, et al. Tissue engineering of cardiac valve prostheses I: development and histological characterization of an acellular porcine scaffold. J Heart Valve Dis 2002;11:457-62. [PubMed]
- Korossis SA, Booth C, Wilcox HE, et al. Tissue engineering of cardiac valve prostheses II: biomechanical characterization of decellularized porcine aortic heart valves. J Heart Valve Dis 2002;11:463-71. [PubMed]
- Kasimir MT, Rieder E, Seebacher G, et al. Comparison of different decellularization procedures of porcine heart valves. Int J Artif Organs 2003;26:421-7. [Crossref] [PubMed]
- Spina M, Ortolani F, El Messlemani A, et al. Isolation of intact aortic valve scaffolds for heart-valve bioprostheses: extracellular matrix structure, prevention from calcification, and cell repopulation features. J Biomed Mater Res A 2003;67:1338-50. [Crossref] [PubMed]
- Tudorache I, Cebotari S, Sturz G, et al. Tissue engineering of heart valves: biomechanical and morphological properties of decellularized heart valves. J Heart Valve Dis 2007;16:567-73; discussion 573-4. [PubMed]
- Lim HG, Kim SH, Choi SY, et al. Anticalcification effects of decellularization, solvent, and detoxification treatment for genipin and glutaraldehyde fixation of bovine pericardium. Eur J Cardiothorac Surg 2012;41:383-90. [Crossref] [PubMed]
- Gonçalves AC, Griffiths LG, Anthony RV, et al. Decellularization of bovine pericardium for tissue-engineering by targeted removal of xenoantigens. J Heart Valve Dis 2005;14:212-7. [PubMed]
- Kasimir MT, Rieder E, Seebacher G, et al. Presence and elimination of the xenoantigen gal (alpha1, 3) gal in tissue-engineered heart valves. Tissue Eng 2005;11:1274-80. [Crossref] [PubMed]
- Naso F, Gandaglia A, Iop L, et al. First quantitative assay of alpha-Gal in soft tissues: presence and distribution of the epitope before and after cell removal from xenogeneic heart valves. Acta Biomater 2011;7:1728-34. [Crossref] [PubMed]
- Simon P, Kasimir MT, Seebacher G, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg 2003;23:1002-6. [Crossref] [PubMed]
- Cicha I, Rüffer A, Cesnjevar R, et al. Early obstruction of decellularized xenogenic valves in pediatric patients: involvement of inflammatory and fibroproliferative processes. Cardiovasc Pathol 2011;20:222-31. [Crossref] [PubMed]
- Sievers HH, Stierle U, Schmidtke C, et al. Decellularized pulmonary homograft (SynerGraft) for reconstruction of the right ventricular outflow tract: first clinical experience. Z Kardiol 2003;92:53-9. [Crossref] [PubMed]
- Brown JW, Elkins RC, Clarke DR, et al. Performance of the CryoValve SG human decellularized pulmonary valve in 342 patients relative to the conventional CryoValve at a mean follow-up of four years. J Thorac Cardiovasc Surg 2010;139:339-48. [Crossref] [PubMed]
- da Costa FD, Costa AC, Prestes R, et al. The early and midterm function of decellularized aortic valve allografts. Ann Thorac Surg 2010;90:1854-60. [Crossref] [PubMed]
- Cebotari S, Tudorache I, Ciubotaru A, et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation 2011;124:S115-23. [Crossref] [PubMed]
- Kneib C, von Glehn CQ, Costa FD, et al. Evaluation of humoral immune response to donor HLA after implantation of cellularized versus decellularized human heart valve allografts. Tissue Antigens 2012;80:165-74. [Crossref] [PubMed]
- Ruzmetov M, Shah JJ, Geiss DM, et al. Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: a single-institution comparison. J Thorac Cardiovasc Surg 2012;143:543-9. [Crossref] [PubMed]
- Tudorache I, Horke A, Cebotari S, et al. Decellularized aortic homografts for aortic valve and aorta ascendens replacement. Eur J Cardiothorac Surg 2016;50:89-97. [Crossref] [PubMed]
- Zünd G, Hoerstrup SP, Schoeberlein A, et al. Tissue engineering: a new approach in cardiovascular surgery: Seeding of human fibroblasts followed by human endothelial cells on resorbable mesh. Eur J Cardiothorac Surg 1998;13:160-4. [Crossref] [PubMed]
- Shinoka T. Tissue engineered heart valves: autologous cell seeding on biodegradable polymer scaffold. Artif Organs 2002;26:402-6. [Crossref] [PubMed]
- Bertipaglia B, Ortolani F, Petrelli L, et al. Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: the VESALIO Project (Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur). Ann Thorac Surg 2003;75:1274-82. [Crossref] [PubMed]
- Rieder E, Kasimir MT, Silberhumer G, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg 2004;127:399-405. [Crossref] [PubMed]
- Knight RL, Booth C, Wilcox HE, et al. Tissue engineering of cardiac valves: re-seeding of acellular porcine aortic valve matrices with human mesenchymal progenitor cells. J Heart valve Dis 2005;14:806-13. [PubMed]
- Iop L, Renier V, Naso F, et al. The influence of heart valve leaflet matrix characteristics on the interaction between human mesenchymal stem cells and decellularized scaffolds. Biomaterials 2009;30:4104-16. [Crossref] [PubMed]
- Tedder ME, Simionescu A, Chen J, et al. Assembly and testing of stem cell-seeded layered collagen constructs for heart valve tissue engineering. Tissue Eng Part A 2011;17:25-36. [Crossref] [PubMed]
- Albanna MZ, Bou-Akl TH, Walters HL 3rd, et al. Improving the mechanical properties of chitosan-based heart valve scaffolds using chitosan fibers. J Mech Behav Biomed Mater 2012;5:171-80. [Crossref] [PubMed]
- Weymann A, Schmack B, Okada T, et al. Reendothelialization of human heart valve neoscaffolds using umbilical cord-derived endothelial cells. Circ J 2013;77:207-16. [Crossref] [PubMed]
- Eslami M, Vrana NE, Zorlutuna P, et al. Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J Biomater Appl 2014;29:399-410. [Crossref] [PubMed]
- Santoro R, Consolo F, Spiccia M, et al. Feasibility of pig and human-derived aortic valve interstitial cells seeding on fixative-free decellularized animal pericardium. J Biomed Mater Res B Appl Biomater 2016;104:345-56. [Crossref] [PubMed]
- Roosens A, Asadian M, De Geyter N, et al. Complete static repopulation of decellularized porcine tissues for heart valve engineering: an in vitro study. Cells Tissues Organs 2017;204:270-82. [Crossref] [PubMed]
- Hoerstrup SP, Kadner A, Melnitchouk S, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation 2002;106:I143-50. [PubMed]
- Schenke-Layland K, Optiz F, Gross M, et al. Complete dynamic repopulation of decellularized heart valves by application of defined physical signals-an in vitro study. Cardiovasc Res 2003;60:497-509. [Crossref] [PubMed]
- Lichtenberg A, Tudorache I, Cebotari S, et al. In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials 2006;27:4221-9. [Crossref] [PubMed]
- Sodian R, Lueders C, Kraemer L, et al. Tissue engineering of autologous human heart valves using cryopreserved vascular umbilical cord cells. Ann Thorac Surg 2006;81:2207-16. [Crossref] [PubMed]
- Schmidt D, Mol A, Breymann C, et al. Living autologous heart valves engineered from human prenatally harvested progenitors. Circulation 2006;114:I125-31. [Crossref] [PubMed]
- Flanagan TC, Cornelissen C, Koch S, et al. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials 2007;28:3388-97. [Crossref] [PubMed]
- Lee DJ, Steen J, Jordan JE, et al. Endothelialization of heart valve matrix using a computer-assisted pulsatile bioreactor. Tissue Eng Part A 2009;15:807-14. [Crossref] [PubMed]
- Ramaswamy S, Gottlieb D, Engelmayr GC, et al. The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells. Biomaterials 2010;31:1114-25. [Crossref] [PubMed]
- Ghodsizad A, Bordel V, Wiedensohler H, et al. Magnetically guided recellularization of decellularized stented porcine pericardium-derived aortic valve for TAVI. ASAIO J 2014;60:582-6. [Crossref] [PubMed]
- Dohmen PM, Costa Fd, Lopes SV, et al. Results of a decellularized porcine heart valve implanted into the juvenile sheep model. Heart Surg Forum 2005;8:E100-4; discussion E104.
- Baraki H, Tudorache I, Braun M, et al. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials 2009;30:6240-6. [Crossref] [PubMed]
- Honge JL, Funder J, Hansen E, et al. Recellularization of aortic valves in pigs. Eur J Cardiothorac Surg 2011;39:829-34. [Crossref] [PubMed]
- Della Barbera M, Valente M, Basso C, et al. Morphologic studies of cell endogenous repopulation in decellularized aortic and pulmonary homografts implanted in sheep. Cardiovasc Pathol 2015;24:102-9. [Crossref] [PubMed]
- Tudorache I, Theodoridis K, Baraki H, et al. Decellularized aortic allografts versus pulmonary autografts for aortic valve replacement in the growing sheep model: haemodynamic and morphological results at 20 months after implantation. Eur J Cardiothorac Surg 2016;49:1228-38. [Crossref] [PubMed]
- Hennessy RS, Go JL, Hennessy RR, et al. Recellularization of a novel off-the-shelf valve following xenogenic implantation into the right ventricular outflow tract. PLoS One 2017;12:e0181614. [Crossref] [PubMed]
- Crick SJ, Sheppard MN, Ho SY, et al. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat 1998;193:105-19. [Crossref] [PubMed]
- Verdouw PD, van den Doel MA, de Zeeuw S, et al. Animal models in the study of myocardial ischaemia and ischaemic syndromes. Cardiovasc Res 1998;39:121-35. [Crossref] [PubMed]
- Lelovas PP, Kostomitsopoulos NG, Xanthos TT. A comparative anatomic and physiologic overview of the porcine heart. J Am Assoc Lab Anim Sci 2014;53:432-8. [PubMed]
- Schuleri KH, Boyle AJ, Centola M, et al. The adult Göttingen minipig as a model for chronic heart failure after myocardial infarction: focus on cardiovascular imaging and regenerative therapies. Comp Med 2008;58:568-79. [PubMed]
- Numata S, Fujisato T, Niwaya K, et al. Immunological and histological evaluation of decellularized allograft in a pig model: comparison with cryopreserved allograft. J Heart Valve Dis 2004;13:984-90. [PubMed]
- Gallo M, Naso F, Poser H, et al. Physiological performance of a detergent decellularized heart valve implanted for 15 months in Vietnamese pigs: surgical procedure, follow-up, and explant inspection. Artif Organs 2012;36:E138-50. [Crossref] [PubMed]
- Iop L, Bonetti A, Naso F, et al. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS One 2014;9:e99593. [Crossref] [PubMed]
- Gallo M, Poser H, Bottio T, et al. The Vietnamese pig as a translational animal model to evaluate tissue engineered heart valves: promising early experience. Int J Artif Organs 2017;40:142-9. [Crossref] [PubMed]
- Gallo M, Bonetti A, Poser H, et al. Decellularized aortic conduits: could their cryopreservation affect post-implantation outcomes? A morpho-functional study on porcine homografts. Heart Vessels 2016;31:1862-73. [Crossref] [PubMed]