Heart-lung transplantation
Introduction
Experimentation in heart-lung transplantation was conducted for more than 25 years prior to the first clinical success (1,2). The initial studies in dogs were marked by failures related to altered respiratory pattern in these animals, most likely a consequence of cardiopulmonary denervation. This was not seen when these experiments were performed in primates (3,4). In the late 1960’s and early 1970’s three attempts at heart-lung transplantation were made. The longest survival of these three was 23 days. Finally in the early 1980’s, the group in Stanford successfully transplanted the heart and lungs into three recipients all of whom had pulmonary vascular disease (5). Two of the three were long term survivors of greater than 5 years. The introduction of cyclosporine as an immunosuppressant was felt to be integral in these successful transplants. These patients actually represent the first long term survivors of any sort of lung transplant as clinical isolated lung transplants did not occur until 4-5 years subsequent to this (6). Initially, the majority of heart-lung transplants were for pulmonary vascular disease and cystic fibrosis, diseases primarily treated with lung transplantation alone currently. Fewer and fewer heart-lung transplants have been performed since 1990 when the largest number were recorded in the registry maintained by the International Society of Heart and Lung Transplantation. Less than 100 are performed throughout the world today (7). The role of heart-lung transplantation continues to evolve. Technical problems account for approximately one-fifth of all deaths early following heart-lung transplantation, hence the importance of having a firm grasp on the surgical technique of both the harvest and organ implant.
Donor evaluation and harvest
The donor organs individually must meet the same criteria for donation as for isolated heart and lung transplantation. The heart function must be nearly normal on modest inotropic support at most. There should be no significant valvar stenosis or insufficiency. The chest radiograph should be free of significant infiltrates and the arterial pO2 on oxygen challenge should exceed 350 mmHg. The donor must be free of systemic infection and have no evidence of malignancy. Size matching is often difficult because of the relative malnourished state of recipients with end-stage heart and lung disease. A larger donor may be problematic fitting the organs into the chest of the recipient unless there is significant hyperexpansion of the lungs creating a larger thoracic cavity. Recipients with fibrotic lung diseases typically have contracted chest cavities; one should be very cautious of a larger donor in these instances. The lungs can be trimmed or a lobectomy performed to allow for a better fit in some cases. Smaller donors obviously will fit easily but potentially can suffer hyperexpansion pulmonary edema when the mismatch is significant. In general, one is safe to accept a donor 10% above and below the weight of the recipient with a similar height range. Beyond this very limited range, one can expand the accepted donor size based upon the recipient characteristics.
The final evaluation of the donor is on-site with flexible bronchoscopy to evaluate the airways for evidence of aspiration or pneumonia as well as looking for other anomalies. A median sternotomy is performed. The donor heart is examined by direct inspection with the chest open. The pleural spaces are opened widely to allow direct visual and tactile examination of the lungs. The trachea is dissected circumferentially between the aorta and the superior vena cava. Both the superior vena cava and inferior vena cava are dissected out. At the appropriate time, heparin is given intravenously and prostaglandin E1 is administered into the main pulmonary artery. The inferior vena cava is divided and the left atrial appendage is amputated. This allows complete emptying of the heart. The aorta is cross-clamped and both the heart preservative and lung preservative solutions are delivered via cannulae inserted into the ascending aorta and main pulmonary artery respectively. Topical cold saline and slush are applied to the organs. A nominal ventilator rate should be maintained throughout this period of time to enhance the distribution of the pulmoplegia.
The organs are harvested as a heart-lung bloc. The pericardium is divided down to the diaphragm and posteriorly along the diaphragm. The inferior pulmonary ligaments are divided up to the inferior pulmonary veins on each side. The left lung is flipped medially, effectively out of the pleural space allowing access to the posterior mediastinum. The pleura there are divided with a knife and the mediastinal contents are bluntly mobilized including the esophagus and descending aorta. A similar procedure is performed in the right pleural space. The aorta is divided at the level of the innominate artery; a longer segment of aorta can be taken if necessary for any reconstructive purposes in the recipient. The trachea is mobilized further and stapled to occlude it distally at least one centimeter above the carina. The lungs should be mildly inflated at low pressure at the time of application of the stapler. It is then divided proximally while occluded with a clamp of some sort. The esophagus is divided with a GIA type of stapler proximally and distally. The NG tube should have been removed and the endotracheal tube pulled back enough to be excluded from the stapling devices. The descending thoracic aorta is divided. The heart-lung bloc can now be removed from the chest and placed in cold solution, usually the cardioplegia solution, and then placed in cold storage for transport.
Recipient operation
Preparation of the heart-lung bloc
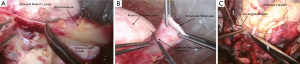
The heart-lung bloc is taken out of cold storage at the appropriate time and all excess mediastinal tissue is removed (Figure 1). This includes the mediastinal portion of the esophagus, in addition to the excess aorta and pericardium (Figure 2A). The paratracheal tissue of the donor should be left intact to facilitate post-transplant blood supply to the area of the anastomosis. This comes primarily from coronary artery collaterals. The staple line on the trachea is removed leaving one or two cartilaginous rings above the take-off of the right mainstem bronchus for the tracheal anastomosis (Figure 2B). A culture of the tracheal secretions is taken and all the retained mucous is suctioned with a separate suction device which will be discarded as soon as the tracheal anastomosis is completed. The amputated left atrial appendage is closed with a pursestring stitch (Figure 2C) which is placed on a tourniquet for use during the transplant procedure. This is done while preparing to perform the aortic anastomosis during the transplant procedure. The atrial septum is inspected through the orifice of the inferior vena cava and any defect present should be closed at this point.

Cardiopneumonectomy
In general, a median sternotomy is the optimal approach for heart-lung transplantation. Given the circumstances, one should perform as much of the dissection as possible prior to initiating cardiopulmonary bypass. This is particularly true for patients who have had prior operations. The pleural spaces are opened widely and all adhesions are taken down. Care is taken to preserve the phrenic nerves. The pericardium is opened posterior to the right phrenic nerve as far from the nerve as feasible. The donor lung will be placed posterior to the nerves to get into the respective pleural spaces, so this posterior opening from the pericardium into the pleural space must be along nearly all the length of the phrenic nerves.
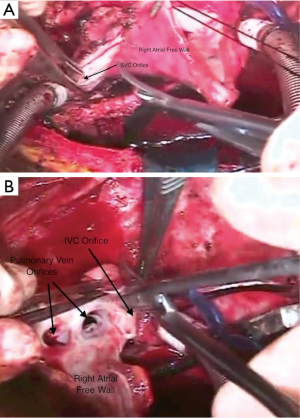
The patient is then placed on cardiopulmonary bypass using bi-caval cannulation and cooled to 28 degrees C (Figure 3). The cavae are snared and the aorta cross-clamped. The heart is then excised followed by each lung. One can anticipate significant pulmonary venous return via the extensive aortopulmonary collateral network that commonly accompanies patients with the diseases for which heart-lung transplantation is performed. The aorta is divided just above the aortic valve and the main pulmonary artery just above the pulmonic valve. The right atrium is opened with the incision going onto the roof around the right atrial appendage and down toward the coronary sinus. The incision in the roof of the right atrium is taken across into the left atrium and then follows along the atrio-ventricular groove. The atrial septum is divided down toward the coronary sinus. The heart is then removed from the field. Excess right atrium is removed, leaving sufficient cuffs of tissue for the superior and inferior venae cavae anastomoses.

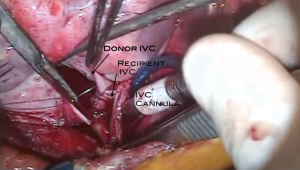
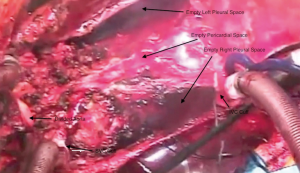
Bilateral pneumonectomies are then performed (Figure 4). Each lung is dissected out of the pleural space leaving only the bronchus attached. The pulmonary artery and vein branches do not have to be ligated, but rather can be divided with the electrocautery. The mainstem bronchus is then stapled and the distal bronchus divided. The lungs are then removed from the thoracic cavity. The excess atrial tissue is removed along with any remnants of the proximal branch pulmonary arteries. A sufficient rim of inferior and superior vena cava is necessary for the respective connections with the donor heart (Figures 5,6). It is generally advisable to leave a small island of pulmonary artery at the insertion of the ligamentum arteriosum so that risk of injury to the left recurrent laryngeal nerve is lessened. Both mainstem bronchi are then grasped with Allis clamps to assist with the remaining dissection of the distal airway (Figure 7). A stay suture is placed on the more proximal trachea for traction. The trachea is then divided just above the takeoff of the right mainstem bronchus, which is usually slightly more cephalad than the left. The final step in this portion of the operation is meticulous hemostasis. There are often many mediastinal collateral and bronchial vessels which can cause vexing problems with bleeding if not addressed at this point where exposure is optimal. With both lungs and the heart out of the chest, there is an impressive cavity left behind (Figure 8).



Transplant procedure
The heart-lung bloc is then lowered into the chest cavity passing the left lung posterior to the phrenic nerve/pericardial pedicle and then the right lung into the left chest posterior to the left phrenic nerve pedicle (Figure 9). The order of which lung is passed first is not important. This should place the heart in the midline, lining up the trachea for its anastomotic connection to the recipient trachea. The tracheal anastomosis is done first using a running polypropylene suture (Figures 10,11). Some surgeons prefer running the membranous portion and interrupting the cartilaginous portion. When this anastomosis is completed it should be wrapped with whatever viable tissue is in the vicinity, such as pericardium or lymphatic tissue so that the suture line is not up against a vascular structure. This may also provide some additional security against ischemia at the level of the anastomosis. At this point, a catheter is placed into the left atrium via the appendage using the pursestring stitch placed around the amputated left atrial appendage during the preparation of the heart-lung bloc (Figure 12). This catheter can be a small vent. It is used to infuse cold crystalloid solution. This keeps the heart cool, but also serves as a way to evacuate air from the left sided cardiac structures because there is no pulmonary venous return at all during the organ implant of a heart-lung transplant. Next the aortic anastomosis is performed (Figures 13,14). As this is being completed the cold saline infusing into the left atrium will be coming out the aorta. The cross clamp is then removed and the saline infusion is stopped. The catheter inserted via the left atrial appendage can now be converted to a vent. The inferior vena cava anastomosis is performed next (Figures 15,16). Alternatively, this could be done with the aortic cross clamp still on; this avoids the nuisance of the coronary sinus blood flooding the operative field. However, it does extend the ischemic time somewhat. The superior vena cava anastomosis is then performed (Figure 17). Care must be taken to avoid pursestringing this anastomosis.

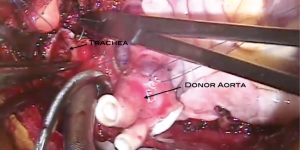





With the completion of all the connections for the new heart-lung bloc, time is taken while on cardiopulmonary bypass to ensure hemostasis. This cannot be emphasized enough. The tissues incised in the process of the recipient cardiectomy and pneumonectomies are all vascular, especially in the setting of cyanotic congenital heart disease or when there have been previous chest operations. Bronchial arteries as well as arterial supply to lymphatic tissue are all large and may be difficult to control with the electrocautery alone. This is all performed while on cardiopulmonary bypass to allow manipulation of the heart and lungs to visualize those areas that would otherwise be difficult to see. Ventilation is then initiated and the patient is weaned from cardiopulmonary bypass (Figure 18).

Special considerations
Patients with congenital heart disease often present anatomic challenges when isolated heart transplantation is to be performed. Many of these challenges are eliminated by virtue of the complete evacuation of all mediastinal and chest contents to implant the heart-lung bloc. However, there are some situations that are worthy of mention.
Systemic venous anomalies that might be encountered include bilateral superior venae cavae, interrupted inferior vena cava with azygous continuation to the superior vena cava or hemiazygous continuation to the left superior vena cava, isolated hepatic veins entering directly into the right atrium. In general, all of these entities are best handled by maintaining the route of venous return to the right atrium and performing an atrial anastomosis rather than caval anastomoses. The left superior vena cava returns blood to the right atrium via the coronary sinus. When the recipient cardiectomy is performed the coronary sinus is left intact by trimming off the heart above the coronary sinus at the level of the atrioventricular grove. Azygous continuation of an interrupted inferior vena cava results in a very large superior vena cava that will likely have a significant size mismatch with the donor superior vena cava. Depending upon the size discrepancy, the more practical approach to this may be an atrial anastomosis rather than caval anastomoses.
Situs inversus is another entitiy producing challenges in technical management. Since there is no left atrial anastomosis in heart-lung transplantation, the entire atrial mass can be devoted to the right atrial anastomosis. When the recipient cardiectomy is performed, the atrial septum is removed. A portion of the wall of the anatomic right atrium on the patient’s left side is closed, effectively moving the atrial anastomosis to the right, using the recipient anatomic left atrium. The right lung of the donor heart-lung bloc must pass under this atrial mass from left to right to obtain optimal positioning.
Summary
Heart-lung transplant is a procedure performed infrequently even in centers with large heart and lung transplant programs. Those patients often have complex problems that make isolated heart or lung transplant not possible. It is critical that recipients be carefully chosen and that all aspects of the transplant procedure be carefully planned in advance, especially for recipients with congenital cardiac anomalies and have had prior palliative operations. These challenging patients require experienced congenital heart surgeons with expertise in heart-lung transplantation to ensure optimal utilization of these precious organs.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Webb WR, Howard HS. Cardio-pulmonary transplantation. Surg Forum 1957;8:313-7. [PubMed]
- Lower RR, Stofer RC, Hurley EJ, et al. Complete homograft replacement of the heart and both lungs. Surgery 1961;50:842-5. [PubMed]
- Nakae S, Webb WR, Theodorides T, et al. Respiratory function following cardiopulmonary denervation in dog, cat, and monkey. Surg Gynecol Obstet 1967;125:1285-92. [PubMed]
- Reitz BA, Burton NA, Jamieson SW, et al. Heart and lung transplantation: autotransplantation and allotransplantation in primates with extended survival. J Thorac Cardiovasc Surg 1980;80:360-72. [PubMed]
- Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med 1982;306:557-64. [PubMed]
- Patterson GA, Cooper JD, Dark JH, et al. Experimental and clinical double lung transplantation. J Thorac Cardiovasc Surg 1988;95:70-4. [PubMed]
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. [PubMed]
- Huddleston CB, Richey SR. Preparation of the heart-lung bloc. Asvide 2014;1:248. Available online: http://www.asvide.com/articles/260
- Huddleston CB, Richey SR. Recipient cardiectomy. Asvide 2014;1:249. Available online: http://www.asvide.com/articles/261
- Huddleston CB, Richey SR. Left pneumonectomy. Asvide 2014;1:250. Available online: http://www.asvide.com/articles/262
- Huddleston CB, Richey SR. Preparation of the IVC and SVC. Asvide 2014;1:251. Available online: http://www.asvide.com/articles/263
- Huddleston CB, Richey SR. Removal of bronchi and distal trachea. Asvide 2014;1:252. Available online: http://www.asvide.com/articles/264
- Huddleston CB, Richey SR. Placement of the heart-lung bloc. Asvide 2014;1:253. Available online: http://www.asvide.com/articles/265
- Huddleston CB, Richey SR. Tracheal anastomosis. Asvide 2014;1:254. Available online: http://www.asvide.com/articles/266
- Huddleston CB, Richey SR. Placement of the LA catheter. Asvide 2014;1:255. Available online: http://www.asvide.com/articles/267
- Huddleston CB, Richey SR. Aortic anastomosis. Asvide 2014;1:256. Available online: http://www.asvide.com/articles/268
- Huddleston CB, Richey SR. IVC anastomosis. Asvide 2014;1:257. Available online: http://www.asvide.com/articles/269
- Huddleston CB, Richey SR. SVC anastomosis. Asvide 2014;1:258. Available online: http://www.asvide.com/articles/270
- Huddleston CB, Richey SR. Functioning transplanted heart and lungs. Asvide 2014;1:259. Available online: http://www.asvide.com/articles/271