
Intermittent pneumatic compression on top of pharmacological thromboprophylaxis in intensive care: added value or added cost?
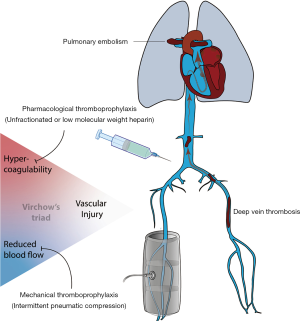
Venous thromboembolism (VTE) is a leading cause of in-hospital morbidity and mortality, particularly in patients at intensive care units (ICU) (1). Hypercoagulability, venous stasis and vascular injury, but also the frequent use of highly coagulable catheters and extracorporeal support devices predispose this patient group to thromboembolic complications. Therefore, efficient strategies for the prevention of thrombosis that do not cause excessive bleeding are paramount in critically ill patients.
In view of the multi-causal pathogenesis of venous thrombosis, both pharmacologic strategies with anticoagulant drugs and mechanical strategies with devices that decrease lower-limb venous stasis by blood displacing (2) reduce the rate of VTE and have been extensively evaluated in both surgical and non-surgical patients. An overview of current recommended strategies for in hospital thromboprophylaxis is provided in Table 1 and Figure 1.
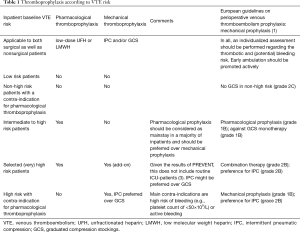
Full table
According to the guidelines of the American College of Chest Physicians, pharmacologic thromboprophylaxis with low-molecular-weight-heparin (LMWH) or low-dose unfractionated heparin (UFH) is the recommended strategy in ICU-patients, even though the number of well-performed randomized clinical trials are limited (4). Mechanical alternatives, with IPC preferred over graduated compression stockings, are first choice in patients at very high risk for VTE who have contra-indications for prophylactic anticoagulant therapy (e.g., active bleeding or high risk for major bleeding) (5,6). Indeed, the difficult balance between bleeding and thrombosis is a daily struggle for physicians working at ICU-departments. Hence, institutional protocols regarding thromboprophylaxis should include both pharmacological and mechanical strategies, each with their own merits and risks (1,4,7,8).
Although pharmacologic thromboprophylaxis reduced the incidence of deep-vein thrombosis by 50% compared with no prophylaxis in critically ill patients, still 5% to 20% of this pharmacologically treated patient group develops deep-vein thrombosis (6,9,10). However, no previous clinical trials addressed whether IPC provides a meaningful benefit when added on top of anticoagulant prophylaxis therapy in critically ill patients. Vice versa, no clinical trials in this patient group evaluated whether pharmacological prophylaxis on top of IPC reduces clinically meaningful thrombotic events.
Therefore, Arabi et al. investigated if adjunctive IPC reduced the risk of VTE in critically ill patients receiving pharmacologic thromboprophylaxis (3). This is an important question because such combined strategy has been implemented in many critical care units in spite of absence of clinical data. Moreover, IPC immobilizes patients yet mobilizes precious resources at ICU (nursing time and cost of goods), and may cause some skin-injuries.
The PREVENT (The Pneumatic Compression for Preventing Venous Thromboembolism) trial was an international, randomized controlled trial in a heterogeneous group of 2003 medical, surgical and trauma intensive care patients, although the latter only represented 8% of the study population. The trial compared IPC for at least 18 hours per day in addition to pharmacologic thromboprophylaxis (LMWH or UFH) with pharmacologic thromboprophylaxis alone. The trial was not double blind (for obvious reasons), but the risk of bias was mitigated because of its randomized design, high adherence to the assigned treatment (22 hours of IPC per day, median duration of 7 days), low incidence of concomitant use of compression stockings (0.9%) and minimal loss to follow-up (3%). Although the trial is underpowered as a result of the lower incidence of DVT in both groups (approximately one third of the predicted primary outcome events were achieved), the event rates in both groups were nearly identical (3.9% in the IPC-arm vs. 4.2% in the control; P=ns).
In conclusion, the PREVENT-trial showed that the routine use of adjunctive IPC did not provide a clinically meaningful effect on the incidence of proximal DVT in critically ill patients at ICU (mean APACHE-II 20) who were concomitantly receiving pharmacologic thromboprophylaxis. The PREVENT-trial will help to refine international guidelines and institutional protocols on VTE thromboprophylaxis, especially in the money saving, time consuming and protocol driven environment of critical care (11-14).
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Verhamme reports grants and personal fees from Bayer Healthcare, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Pfizer, grants and personal fees from BMS, grants and personal fees from Daiichi-Sankyo, grants and personal fees from Leo Pharma, personal fees from Portola and Medtronic, outside the submitted work. The other authors have no conflicts of interest to declare.
References
- Afshari A, Fenger-Eriksen C, Monreal M, et al. European guidelines on perioperative venous thromboembolism prophylaxis: Mechanical prophylaxis. Eur J Anaesthesiol 2018;35:112-5. [PubMed]
- Chen AH, Frangos SG, Kilaru S, et al. Intermittent pneumatic compression devices -- physiological mechanisms of action. Eur J Vasc Endovasc Surg 2001;21:383-92. [Crossref] [PubMed]
- Arabi YM, Al-Hameed F, Burns KEA, et al. Adjunctive Intermittent Pneumatic Compression for Venous Thromboprophylaxis. N Engl J Med 2019;380:1305-15. [Crossref] [PubMed]
- Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:7S-47S.
- Dennis M, Sandercock P, Graham C, et al. The Clots in Legs Or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess 2015;19:1-90. [Crossref] [PubMed]
- Arabi YM, Khedr M, Dara SI, et al. Use of intermittent pneumatic compression and not graduated compression stockings is associated with lower incident VTE in critically ill patients: a multiple propensity scores adjusted analysis. Chest 2013;144:152-9. [Crossref] [PubMed]
- Sachdeva A, Dalton M, Lees T. Graduated compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev 2018;11:CD001484. [PubMed]
- Treasure T, Hill J. NICE guidance on reducing the risk of venous thromboembolism in patients admitted to hospital. J R Soc Med 2010;103:210-2. [Crossref] [PubMed]
- Alhazzani W, Lim W, Jaeschke RZ, et al. Heparin thromboprophylaxis in medical-surgical critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care Med 2013;41:2088-98. [Crossref] [PubMed]
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999;341:793-800. [Crossref] [PubMed]
- Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended Thromboprophylaxis with Betrixaban in Acutely Ill Medical Patients. N Engl J Med 2016;375:534-44. [Crossref] [PubMed]
- Cohen AT, Spiro TE, Buller HR, et al. Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis 2011;31:407-16. [Crossref] [PubMed]
- Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med 2011;365:2167-77. [Crossref] [PubMed]
- Hull RD, Schellong SM, Tapson VF, et al. Extended-duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study. J Thromb Thrombolysis 2006;22:31-8. [Crossref] [PubMed]


