Transcutaneous PCO2 monitoring in critically ill patients: update and perspectives
Introduction
The measurement of oxygen (O2) and carbon dioxide (CO2) gas tension via a transcutaneous route which could non-invasively assess arterial blood gas pressures (artPO2 and artPCO2, respectively) was developed in the 1980s (1). For transcutaneous capnometry (measuring transcutaneous carbon dioxide gas pressure, tcPCO2), sensors are based on chemical electrodes, which Dr. Severinghaus adapted for use in blood gas analysis (2-4). In respiratory failure, the evaluation of adequacy of alveolar ventilation with artPCO2 remains a routine challenge. With consideration of some technical or device-related cautions, relevant interpretation of tcPCO2 measurement is plausible, and can lead to reliable artPCO2 estimation with transcutaneous capnometry monitoring (TCM) while limiting blood gas analysis and arterial puncture (5). Importantly, tcPCO2 is also by nature and physiology a circulatory variable which is dependent on systemic and local cutaneous perfusion conditions. During circulatory failure, decoupling between artPCO2 and tcPCO2 occurs, leading to tissue hypercarbia unrelated to arterial PCO2 (6-8). Interestingly, this mismatch, with a strong physiological and clinical background, offers potential perspectives for peripheral tissue perfusion monitoring in the critically ill patient (9). Although this approach has been investigated since the 1980s, adherence remains low in daily clinical practice. Updated technology and recent clinical reports of innovative modifications including measurement at low temperature (37 °C) and/or with thermal challenge (electrode heated from 37 °C to 42 °C) have yielded promising results that may provide crucial support for the use of this tool in the field of peripheral tissue perfusion monitoring (1,10,11).
The body of indications and validation studies in ICUs are summarized in this review to give a panorama of potential applications of TCM in critically ill patients.
tcPCO2 technology
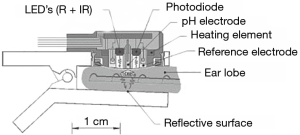
Dr. Severinghaus was the first to describe the measurement of PCO2 on human skin surfaces (3). Transcutaneous measurement of PCO2 is based on the phenomenon of CO2 gas diffusing very easily throughout the body tissue and skin, and can thus be detected by a sensor on the skin surface. CO2 is measured by determining the pH of an electrolyte solution separated from the skin by a highly permeable membrane. A change in the pH is then proportional to the logarithm of PCO2 change (Figure 1). By heating the skin, vasodilation with local hyperemia is produced which increases the diffusion of CO2 and increases the delivery of arterial blood to the dermal capillary area beneath the sensor. Most of the time, the sensor is heated between 42 °C and 44 °C to create the “arterialization” of the tissue (by small arteriole and capillary dilatation) leading to an increase of arterial contribution in the signal. Overall, transcutaneous PCO2 measurements correlate fairly well with the corresponding arterial PCO2 values, even after applying a correcting algorithm to take into consideration the physico-chemical modifications after elevating the temperature of the sensor (2).
This electrochemical method has proven to be accurate and reliable but requires an ex vivo “calibration period” before placing the sensor on the skin, and a subsequent in vivo “equilibration period” to obtain a stable value. It should be noted that the position of the sensor at the earlobe shortens this equilibration time due to its rich vascularization and thus decreases the time response and analytic inertia during acute changes. This technical limitation has hindered the development and use of tcPCO2 monitoring as a surrogate of artPCO2 in current practice (1). A technology based on obtaining tcPCO2 by infrared light is currently being developed to try to increase the ease and reactivity of bedside measurement (2).
tcPCO2 monitoring: physiological overview
Physiology of tissue and cutaneous carbon dioxide monitoring has a long and well-established physiological background, which has been the foundation for the development of different mucosal and cutaneous capnometric devices, extensively described in recent quality reviews (1,6,9). At its core, the measurement of tcPCO2 is dependent on three main phenomena:
- The production of CO2 by the tissues (VCO2);
- The removal of CO2 from the tissues by perfusion (so-called “washout-out” phenomenon);
- The reference value of CO2 at tissue inlet represented by arterial CO2 content (CaCO2).
For this reason, there are, at present, roughly two clinical uses for tcPCO2 measurement: a respiratory use where tcPCO2 is likely to non-invasively estimate and track artPCO2, and a hemodynamic use where tcPCO2 could reflect tissue perfusion by an evaluation of the difference between tcPCO2 and artPCO2, so-called “gap CO2”. The simplified physiology of TCM and the main clinical scenario reflecting these two indications are schematically illustrated in Figure 2 (respiratory use Figure 2A,B, hemodynamic use Figure 2C,D,E,F). Additionally, we have depicted three frequent and relevant clinical issues and described them according to whether the monitoring of tcPCO2 is performed with a sensor at 37 °C or a heated sensor at 42 °C to 44 °C (11,12). The three clinical hemodynamic situations are the following:
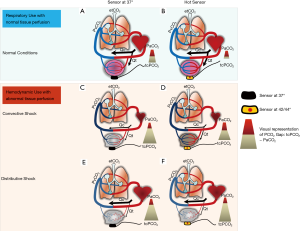
- A stable circulatory state with almost preserved tissue perfusion conditions, when tcPCO2 can be interpreted as an artPCO2 surrogate;
- An overt shock state with low cardiac output or low O2 delivery called “convective shock”, for and corresponding to core pathophysiological patterns of hypovolemic, hemorrhagic or cardiogenic shock;
- A “distributive shock” corresponding to a resuscitated septic shock with restored cardiac output but with an alteration of peripheral microperfusion.
Moreover, as warming the skin impacts tcPCO2 value and local cutaneous blood flow, behavior of tissue hypercarbia depends on locally applied electrode temperature (1,2). For this reason, interpretation of tcPCO2 measurements must take into account the temperature level (i.e., normothermia at 37 °C vs. heated conditions at 42–45 °C) (11). The authors propose this graphic representation in order to illustrate and clarify these six “clinical and measurement situations” based on robust physiological concepts and the results of their recent work. Each illustration will be developed in more detail in the sections to follow.
tcPCO2 monitoring as a surrogate of artPCO2
TCM to track artPCO2 variations: remind the basics
Cutaneous PCO2 represents a mixture of venous, capillary, and arterial CO2 tension values. In normal conditions, tissue metabolism (VCO2) is coupled with tissue perfusion. When metabolism increases, all the CO2 produced is washed out so that the PCO2 gap between tcPCO2 and artPCO2 (tc-artΔPCO2) remains constant at around 5 mmHg (Figure 2A) (8,9). Heating the skin from 37 °C to 45 °C increases the skin blood flow by three to four times and enhances the contribution of arterial blood flow by opening the precapillary sphincter arterioles (1,3). Also, in preserved circulatory conditions, tcPCO2 with heated electrodes (42–45 °C) will closely approximate artPCO2, as heat produces the so-called “arterialization” of local blood flow in the cutaneous area where the sensor is applied (Figure 2B) (13-15). Two correcting factors are then applied to bring the tcPCO2 value close to the value of artPCO2: (I) a fixed correction is removed from the crude tcPCO2 value, as an “aerobic factor”, and, as a consequence, that tissue PCO2 is always physiologically higher than the arterial PCO2 regardless of the quality of tissue arterialization (4.5 mmHg/°C); (II) a Severinghaus constant is applied, due to the increase of tcPCO2 responds to the CO2 production induced by the heat of the sensor, also called the “metabolic constant”, ranging from 5 to 10 mmHg depending on the type of device (2).
Summary of the clinical evidence for tcPCO2 as a reliable artPCO2 surrogate
As the main purpose of this issue concerning CO2-related variables is to focus on hemodynamic management, we will briefly relate and summarize the main clinical data available on TCM for respiratory use.
We can reasonably state that TCM may be useful for non-invasively and continuously estimating actual arterial PCO2, which can be of critical importance during respiratory pump failure leading to alveolar hypoventilation with hypercapnic issues. This tool could prevent the need to perform iterative blood gas analysis and could help to monitor the course of artPCO2 with populations in whom estimates of artPCO2 may guide therapeutic interventions. Different pathophysiological disorders are likely to promote an increase of artPCO2: low alveolar ventilation (with related respiratory acidosis), increased dead space (anatomic or functional), depressed respiratory drive, or bronchial obstructive diseases as acute exacerbation of chronic obstructive pulmonary disease (COPD), especially whose receiving NIV.
While monitoring tcPCO2 is considered as a valid method in routine respiratory care practice for assessing the adequacy of ventilation (16), and the cumulative data available in the specific setting of critically ill patients appears to be substantial, the precision of the technique as an artPCO2 surrogate is still disputed (3,5). Examination of the aggregated literature suggests that accuracy and reliability appear good with limits of agreement in a narrow range for most ICU patients (inside ±5 mmHg and almost all values inside ±10 mmHg) (1,5). However, this opinion is debated, as other authors claim that confidence may be insufficient, as around 20% of the values of arterial-to-transcutaneousPCO2 difference are outside the acceptable range of ±7.5 mmHg (15). There are also numerous reports underscoring the underestimation in the highest artPCO2 values along with other authors who consider the TCM unsuitable or disputable for the emergency room or ICU patients (3,17,18).
As it concerns end tidal CO2 (EtCO2), pragmatically speaking, the relevance of tcPCO2 could be increased with an initial and punctual concomitant arterial blood gas analysis to estimate initial potential gradient, and repeated sequentially so as to not dismiss the distortion with time. Relating to this, Horvath et al. reported good concordance during NIV for ARF and that discordance might have decreased with the initial tc-artΔPCO2 estimate to rule out discrepancy (19). Additionally, Rodriguez et al. reported good correlation in PCO2 data changes (transcutaneous and arterial) over a 17-hour period, and only 20% of the samples had minor changes in opposing directions (13).
Nonetheless, Conway et al. recently pooled the available literature on the accuracy and precision of TCM to offer the most complete picture about this issue in a review with extensive meta-analysis (whole pooled population: 7,021 paired measurements, 2,817 patients in 73 studies; ICU patients: 16 studies (22% of 73 reviewed studies) with n=2,128 measurements; acute respiratory failure, 14 studies, n=993 paired measurements). In the whole population, they concluded that there are substantial differences between tcPCO2 and artPCO2 depending on the technical aspects (17,20,21), such as location site or temperature of electrode, and advocated the ear lobe as the site and a heated electrode of more than 42 °C for the temperature. However, these authors stated that the available literature attests to TCM being an accurate tool to estimate artPCO2 to a clinically acceptable degree in the adult ICU population (22).
Finally, many factors or limitations should be considered when interpreting tcPCO2-observed values as a surrogate of artPCO2. Hasibeder et al. reported that artPCO2 and cardiac output values could only explain 66% of the tcPCO2 value variability, suggesting that many other factors were distorting the concordance between transcutaneous and arterial CO2 in ICUs (23). In our opinion, it would be interesting to further investigate the role of several factors, especially in most hypercapnic critically ill patients with acidosis, to determine the accuracy of tcPCO2 in outlier ICU patients and help in the interpretation of TCM. The most important factors appear to be the technological concerns relating to device performance and differences between monitors (TCM developed by SenTech© or Radiometer©, fiberoptic sensors etc.), location of sensor for measurement, cutaneous adiposity or edema, and of course, disturbed peripheral perfusion by adrenergic tone, drugs, sepsis, shock, fever, etc. However, the respective contribution of each factor may be difficult to capture in ICUs, as outlined by these authors (13).
To conclude this chapter, and in accordance with abundant concordant literature, we advocate the potential use of TCM in ICUs for ventilator management, because of its non-invasiveness, continuous monitoring, and accuracy of the transcutaneous CO2 sensor technology (2,5,13,15,22,24-26). For the longitudinal use as a trending monitor, we support the application of iterative punctual invasive artPCO2 measurements with blood gas analysis to recalibrate and rescale the difference between arterial and transcutaneous PCO2 value, or tc-artΔPCO2 gap (13,22). In this way, TCM could perform an interesting sniffing function over time to track artPCO2 elevation during therapeutic procedures such as prolonged NIV. Finally, in the case of suspected altered tissue perfusion or ongoing shock, tcPCO2 signal may be ambiguous and should be interpreted with caution, as detailed in the next section.
tcPCO2 monitoring as a marker of tissue perfusion
Proof of concept and clinical rationale for use of capnometric data (CO2 derived parameters) in altered tissue perfusion during macrocirculatory or microcirculatory failure have been extensively demonstrated and described (6-8,27-29). These numerous studies clearly demonstrate that the elevation of tissue CO2 is ubiquitous throughout the body in shock states, and is closely related to tissue perfusion alteration. This paradigm has been evidenced by monitoring tissue PCO2 at different clinically available sites including the gastric, buccal, sub-lingual, and thus, the skin level. Schematically, the difference between tcPCO2 and artPCO2 (tc-artΔPCO2 gradient) can increase when circulatory failure or occult microcirculatory shock is ongoing. This may be considered a limitation of the arterial PCO2 estimation technique, and may give an opportunity for hemodynamic assessment in specific clinical situations.
tcPCO2 in low oxygen delivery situations or convective shocks
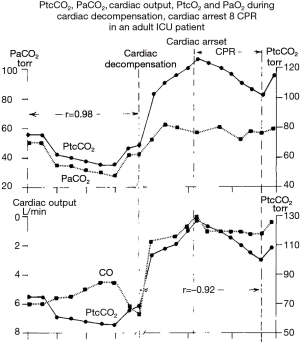
Behavior of tcPCO2 during macrocirculatory failure leading to low cardiac output and/or low O2 delivery (DO2), referred to as “convective shocks” (cardiogenic or hemorrhagic shock), is depicted in (Figure 2C) (12). When circulatory failure occurs, tcPCO2 and artPCO2 mismatch and become decoupled as demonstrated in a famous and seminal clinical study from an L.A. team of Tremper and Shoemaker who monitored the kinetics of tcPCO2 during overt shock states (hemorrhage, heart failure, or the operating room) (Figure 3) (30). This figure illustrates the hemodynamic nature of tcPCO2 as we can observe that tcPCO2 values mirror the cardiac output time course, and become dramatically decoupled from artPCO2 kinetics in the clinical case condition of low flow states. In this setting, note that the difference between tissue and arterial PCO2 is more relevant than the absolute value of tcPCO2 to track local tissue PCO2 balance (and overcome the influence of arterial CO2 content and thus artPCO2 on tcPCO2). In this framework, high PCO2 gap values may be suggestive of flow stagnation by low local perfusion. Many clinical reports, along with robust experimental data, support the notion that hemorrhagic or cardiogenic shocks, together with cardiac arrest, lead to a huge increase in tissue hypercarbia. Of note, some recent additional pre-clinical data reinforce this currently still valid finding (29,31).
tcPCO2 in microcirculatory or distributive shock
According to experts, the gold-standard technique for microcirculatory perturbation assessment remains optical direct sublingual microvideoscopy (SDF-OPS or IDF technologies) (32). However, these tools appear cumbersome, require time-consuming offline analysis, and have not yet reached clinical utility despite over a decade of research and technological advance. Also, a system to assess the microcirculation at the point of care seems highly desirable. On the other hand, the clinical signs of peripheral perfusion impairments (skin mottling, refill capillary time, etc.) are meaningful and informative for microcirculatory derangement but may appear late and be insufficiently sensitive for guiding therapeutics. As outlined by several authors, refined therapeutic tailored management should embrace and target microcirculatory dimensions of shock (33). Tissue capnometry, via gastric or sublingual routes, or more simply with trans-cutaneous monitoring, could aid in this purpose, and offer, as complement, a more sensitive insight than that provided by clinical examination (9-11,27,33).
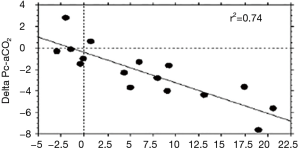
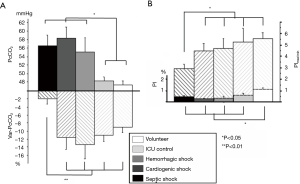
Gastric tonometry and sublingual capnometry have shown their clinical validity and their relationship to microperfusion, but have not been put into practice at this time due to paradigmatic or technological concerns (6,9). As an alternative, skin monitoring at the earlobe thus seems to be a user-friendly way to monitor tissue CO2. Indeed, in a previous work, Vallée et al. used this device to examine whether cutaneous earlobe tcPCO2 could be used to assess tissue perfusion in septic shock patients. In that study, the sensor was heated at 37 °C to limit the impact of arterial PCO2 on cutaneous PCO2 due to the arterialization of the blood being minimal compared with when the sensor is warming to 42 °C (10). They found that a threshold value of 16 mmHg for the gradient between the earlobe tcPCO2 and arterial PCO2 reliably discriminated between those patients with septic shock and tissue hypoperfusion from those patients in the control group, with a sensitivity of 83% and a specificity of 90%. Furthermore, it was found that the fluid challenge induced a decrease in the earlobe to-arterial PCO2 gradient in parallel with the improvement of the microcirculatory blood flow in the earlobe (Figure 4). Interestingly, where a significant reduction in earlobe-to-arterial PCO2 gradient was observed in survivors compared to non-survivors, no significant changes were found with the traditional macrocirculatory parameters (cardiac output and central venous oxygen saturation). Interestingly, these authors confirmed the microcirculatory nature of tcPCO2 signal as demonstrated by the correlation between laser-Doppler flowmetry investigation and tcPCO2 values (Figure 5). tcPCO2 at 37 °C at the earlobe, therefore, seems to be a plausible tool to continuously and non-invasively estimate tissue perfusion in shock patients in ICUs.
tcPCO2 monitoring with variations of sensor temperature: insights from a heat challenge
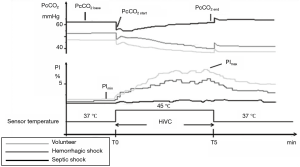
We have seen that the tcPCO2 can be monitored at 37 °C with a heated sensor. The dynamic change in the temperature may therefore appear as a dynamic test to evaluate tissue perfusion during shock. Heat challenge may be added to track microcirculatory failure and reversibility. This concept of studying the variations of cutaneous capnometry during a heating challenge was recently described (Figure 6) (11). The same paradigm has been used in a recent study by the De Backer team with a laser-Doppler flowmetry device, adding external validity for heat or thermal challenge with TCM (34). Schematically, a crude estimate with no heated electrode (standardized normothermia) together with a functional provocative test (as a thermal or heating challenge) could be useful or informative on peripheral perfusion to evaluate tissue hypercarbia related to low flow states or altered microcirculation with loss of “hemodynamic coherence”, as recently conceptualized as occurring during sepsis and “microcirculatory shock” (35). For example, in the case of convective shock, without functional microcirculatory damage, the heat challenge will induce vasodilation which can lead to a decrease in tcPCO2 by a recruited flow (or washout phenomenon) (Figure 2D). This is conceptually more hazardous in the case of a longstanding distributive shock where the constitutive alteration of the microcirculation (shunt, micro-thrombi, etc.) is not even slightly sensitive to vasodilation induced by the local increase in the temperature of the sensor (Figure 2F). Thus, a heat challenge (Figure 2D), which is likely to recruit a microvascular contingent with preserved vasoreactivity, could help to confirm hemodynamic coherence (intact macro-microcirculatory coupling) and/or to diagnose the reversibility of local peripheral hypoperfusion and anticipate targeted therapies (11). From this perspective, the combined monitoring of the perfusion index (PI) from the photoplethysmography signal also allows a good reflection of the quality of vasodilation and “arterialization” induced by the local heating of the sensor (Figure 5).
tcPCO2 monitoring: personal perspectives and unanswered questions
We promote the graphical conceptual framework depicted in Figure 2 to describe two possible uses of TCM in ICUs. The first, and most commonly proposed utility, is when TCM is used to estimate artPCO2 for respiratory issues (Figure 2A,B); the second is when TCM is used to estimate tissue perfusion in shock states (Figure 2C,D,E,F). We believe that these two approaches are not conflicting, but it seems necessary to consider the limitations and specific conditions for each indication. In doing so, we can obtain the appropriate bedside interpretation and receive the maximum benefit from this currently underused, but non-invasive and continuous type of benign monitoring. Indeed, we believe that a dual approach could allow the clinicians to better capture both the respiratory and hemodynamic status of the most severe patients. For example, a patient under respiratory TCM monitoring who exhibits an unexpected increase in tcPCO2 due to de novo or early onset shock, may be misinterpreted as a false positive of a presumed related respiratory issue instead of a tissue perfusion abnormality. For this reason, we might advocate the continuous use of the sensor at low temperature (37 °C) to thereby limit the risk of skin burns, but with regular heating challenges and a coupled and dynamic analysis of all parameters. Indeed, a “normal” 37°-tcPCO2 value would show that there is no patent tissue perfusion disorder (Figure 2A), and then the tcPCO2 value at the end of the heating test would reflect a value close to artPCO2 (Figure 2B). Conversely, a high value of tcPCO2 would attempt to show abnormalities in tissue perfusion (Figure 2C,E), and the heating test would make it possible to monitor the existence of microcirculation dysfunction and its reversibility, which would be strongly related to the prognosis of a patient in shock (Figure 2D,F). We believe that these assumptions would allow for a unique and codified interpretation of TCM. Obviously, additional studies dealing with different clinical situations and populations are mandatory to further support our hypotheses and refine our suggested algorithm. There are also many unanswered questions which include the temperature of the sensor in relation to the skin temperature (iso vs. normothermia), the thermal variations and kinetics during a heating test, the position at the earlobe as a reflection of the whole peripheral perfusion, and the variability and reproducibility of the tcPCO2 value mainly in specific clinical situations such as acidosis or hyperoxia (2,13,23). Furthermore, it will be necessary to compare TCM with other devices that estimate the microcirculation, and to ultimately test drugs targeting microcirculatory dysfunction. To conclude, as a next step, we suggest integrating values of the tcPCO2 and tc-artPCO2 gap into holistic therapeutic algorithms, and advocate considering systemic and regional CO2-related parameters for advanced circulatory monitoring, as recently proposed (36,37).
tcPCO2 monitoring: conclusion
Transcutaneous CO2 monitoring has been developing for many years, and its utility has been proven in at least two different clinical situations in critically ill patients: arterial PCO2 estimation and tissue perfusion monitoring. Probably because of this ambivalence, which can be confusing for clinicians, this monitoring has been, in our opinion, underused thus far. However, recent research has shown that these two aforementioned applications are not irreconcilable and could be combined. We believe that estimating arterial PCO2 and measuring the tcPCO2 gap between arterial-to-tissue CO2, in normothermia (37 °C), combined with the provocative perfusion test as a heat challenge (electrode warmed to 42–44 °C), would help clinicians to continuously and noninvasively capture both respiratory and hemodynamic failures in critically ill patients. Even preliminary, our data on heat challenge as a way to assess microcirculatory shock has shown potential and may stimulate further investigations in this field. For the future, it would be desirable for tcPCO2 sensors to offer refined technological innovation (with automated temperature tests and manipulation of algorithmic constants) in order to popularize the daily use of this device in different clinical settings.
Acknowledgments
None.
Footnote
Conflicts of Interest: A patent application (n° PCT IB2009/006903) is pending on variations of PcCO2 and PI during Heating Challenge. The patent belongs to the Assistance Publique-Hôpitaux de Paris (France). F Vallée and H Nougue received consultant fees from Radiometer. The other authors have no conflicts of interest to declare.
References
- Orbegozo-Cortès D, De Backer D. Transcutaneous O2 and CO2 monitoring. In: Monitoring Tissue Perfusion in Shock. Lima P, Augusto A, Eliézer S (eds). Springer International Publishing AG: 173-80.
- Eberhard P. The design, use, and results of transcutaneous carbon dioxide analysis: current and future directions. Anesth Analg 2007;105:S48-52. [Crossref] [PubMed]
- Huttmann SE, Windisch W, Storre JH. Techniques for the measurement and monitoring of carbon dioxide in the blood. Ann Am Thorac Soc 2014;11:645-52. [Crossref] [PubMed]
- Severinghaus JW, Astrup P, Murray JF. Blood gas analysis and critical care medicine. Am J Respir Crit Care Med 1998;157:S114-22. [Crossref] [PubMed]
- Nassar BS, Schmidt GA. Estimating Arterial Partial Pressure of Carbon Dioxide in Ventilated Patients: How Valid Are Surrogate Measures? Ann Am Thorac Soc 2017;14:1005-14. [Crossref] [PubMed]
- Marik PE. Regional carbon dioxide monitoring to assess the adequacy of tissue perfusion. Curr Opin Crit Care 2005;11:245-51. [Crossref] [PubMed]
- Vallet B, Teboul JL, Cain S, et al. Venoarterial CO(2) difference during regional ischemic or hypoxic hypoxia. J Appl Physiol (1985) 2000;89:1317-21. [Crossref] [PubMed]
- Almac E, Siegemund M, Demirci C, et al. Microcirculatory recruitment maneuvers correct tissue CO2 abnormalities in sepsis. Minerva Anestesiol 2006;72:507-19. [PubMed]
- Mallat J, Vallet B. Mucosal and cutaneous capnometry for the assessment of tissue hypoperfusion. Minerva Anestesiol 2018;84:68-80. [PubMed]
- Vallée F, Mateo J, Dubreuil G, et al. Cutaneous ear lobe Pco2 at 37°C to evaluate microperfusion in patients with septic shock. Chest 2010;138:1062-70. [Crossref] [PubMed]
- Vallée F, Nougué H, Mari A, et al. Variations of Cutaneous Capnometry and Perfusion Index During A Heating Challenge is Early Impaired in Septic Shock and Related to Prognostic in Non-Septic Shock. Shock 2019;51:585-92. [PubMed]
- Weil MH, Shubin H. Proposed reclassification of shock states with special reference to distributive defects. Adv Exp Med Biol 1971;23:13-23. [Crossref] [PubMed]
- Rodriguez P, Lellouche F, Aboab J, et al. Transcutaneous arterial carbon dioxide pressure monitoring in critically ill adult patients. Intensive Care Med. 2006;32:309-12. [Crossref] [PubMed]
- Janssens JP, Howarth-Frey C, Chevrolet JC, et al. Transcutaneous PCO2 to monitor noninvasive mechanical ventilation in adults: assessment of a new transcutaneous PCO2 device. Chest 1998;113:768-73. [Crossref] [PubMed]
- Bendjelid K, Schütz N, Stotz M, et al. Transcutaneous PCO2 monitoring in critically ill adults: clinical evaluation of a new sensor. Crit Care Med 2005;33:2203-6. [Crossref] [PubMed]
- Restrepo RD, Hirst KR, Wittnebel L, et al. AARC clinical practice guideline: transcutaneous monitoring of carbon dioxide and oxygen: 2012. Respir Care 2012;57:1955-62. [Crossref] [PubMed]
- Peschanski N, Garcia L, Delasalle E, et al. Can transcutaneous carbon dioxide pressure be a surrogate of blood gas samples for spontaneously breathing emergency patients? The ERNESTO experience. Emerg Med J 2016;33:325-8. [Crossref] [PubMed]
- Ruiz Y, Farrero E, Córdoba A, et al. Transcutaneous Carbon Dioxide Monitoring in Subjects With Acute Respiratory Failure and Severe Hypercapnia. Respir Care 2016;61:428-33. [Crossref] [PubMed]
- Horvath CM, Brutsche MH, Baty F, et al. Transcutaneous versus blood carbon dioxide monitoring during acute noninvasive ventilation in the emergency department - a retrospective analysis. Swiss Med Wkly 2016;146:w14373. [PubMed]
- Bobbia X, Claret PG, Palmier L, et al. Erratum: Concordance and limits between transcutaneous and arterial carbon dioxide pressure in emergency department patients with acute respiratory failure: a single-center, prospective, and observational study. Scand J Trauma Resusc Emerg Med 2015;23:77. [Crossref] [PubMed]
- Delerme S, Montout V, Goulet H, et al. Concordance between transcutaneous and arterial measurements of carbon dioxide in an ED. Am J Emerg Med 2012;30:1872-6. [Crossref] [PubMed]
- Conway A, Tipton E, Liu WH, et al. Accuracy and precision of transcutaneous carbon dioxide monitoring: a systematic review and meta-analysis. Thorax 2019;74:157-63. [Crossref] [PubMed]
- Hasibeder W, Haisjackl M, Sparr H, et al. Factors influencing transcutaneous oxygen and carbon dioxide measurements in adult intensive care patients. Intensive Care Med 1991;17:272-5. [Crossref] [PubMed]
- Johnson DC, Batool S, Dalbec R. Transcutaneous carbon dioxide pressure monitoring in a specialized weaning unit. Respir Care 2008;53:1042-7. [PubMed]
- Henao-Brasseur J, Bedel J, Mutlu G, et al. Transcutaneous CO2 monitoring: a new tool to identify spontaneous breathing trial failure during weaning from mechanical ventilation. A pilot cohort study. Intensive Care Med 2016;42:1078-9. [Crossref] [PubMed]
- van Oppen JD, Daniel PS, Sovani MP. What is the potential role of transcutaneous carbon dioxide in guiding acute noninvasive ventilation? Respir Care 2015;60:484-91. [Crossref] [PubMed]
- Weil MH, Nakagawa Y, Tang W, et al. Sublingual capnometry: a new noninvasive measurement for diagnosis and quantitation of severity of circulatory shock. Crit Care Med 1999;27:1225-9. [Crossref] [PubMed]
- Tremper KK, Shoemaker WC, Shippy CR, et al. Transcutaneous PCO2 monitoring on adult patients in the ICU and the operating room. Crit Care Med 1981;9:752-5. [Crossref] [PubMed]
- Waelgaard L, Dahl BM, Kvarstein G, et al. Tissue gas tensions and tissue metabolites for detection of organ hypoperfusion and ischemia. Acta Anaesthesiol Scand 2012;56:200-9. [Crossref] [PubMed]
- Tremper KK, Shoemaker WC. Continuous CPR monitoring with transcutaneous oxygen and carbon dioxide sensors. Crit Care Med 1981;9:417-8. [Crossref] [PubMed]
- Belenkiy SM, Berry JS, Batchinsky AI, et al. The noninvasive carbon dioxide gradient (NICO2G) during hemorrhagic shock. Shock 2014;42:38-43. [Crossref] [PubMed]
- Ince C, Boerma EC, Cecconi M, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med 2018;44:281-99. [Crossref] [PubMed]
- Legrand M, Ait-Oufella H, Ince C. Could resuscitation be based on microcirculation data? Yes. Intensive Care Med 2018;44:944-6. [Crossref] [PubMed]
- Orbegozo D, Mongkolpun W, Stringari G, et al. Skin microcirculatory reactivity assessed using a thermal challenge is decreased in patients with circulatory shock and associated with outcome. Ann Intensive Care 2018;8:60. [Crossref] [PubMed]
- Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 2015;19 Suppl 3:S8. [PubMed]
- Perner A, Gordon AC, De Backer D, et al. Sepsis: frontiers in diagnosis, resuscitation and antibiotic therapy. Intensive Care Med 2016;42:1958-69. [Crossref] [PubMed]
- Ospina-Tascón GA, Umaña M, Bermúdez WF, et al. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med 2016;42:211-21. [Crossref] [PubMed]
(English Language Editor: John Ayric Gray, AME Publishing Company)