Adjuvant chemotherapy is associated with improved survival in patients with nodal metastases after neoadjuvant therapy and esophagectomy
Introduction
Deaths attributable to esophageal cancer are expected to top 15,000 in 2018, making esophageal cancer the fifth most common cause of cancer death in the United States (1). Patients frequently present with locoregional advanced disease and have a dismal 20% overall 5-year survival (2,3). Multiple therapeutic strategies have been studied in an effort to extend this survival, but no clear standard regimen exists. For patients with resectable esophageal tumors, neoadjuvant chemoradiation has been associated with improved survival in multiple randomized trials when compared with surgery alone and is currently considered the standard of care (4-6).
The role of adjuvant chemotherapy in patients with esophageal adenocarcinoma is less clear. Retrospective data from a single center suggested that adjuvant chemotherapy is as effective as neoadjuvant chemoradiation therapy, but there are few other studies (7). The potential benefits of adjuvant chemotherapy in patients who received neoadjuvant therapy have not been studied in prospective trials. Current National Comprehensive Cancer Network (NCCN) guidelines recommend either observation until progression or adjuvant chemotherapy for patients with esophageal adenocarcinoma who received preoperative chemoradiation therapy but have persistent nodal disease after resection (8). Evidence for this recommendation is based primarily on a trial of perioperative chemotherapy (9) in which only about half of the patients in the chemotherapy arm received postoperative chemotherapy, and 25% of the patients in the trial had gastric cancers. The aim of the current study was to clarify the role of adjuvant chemotherapy for patients with esophageal adenocarcinoma who are found to have persistent nodal metastases after neoadjuvant chemoradiation and complete resection.
Methods
The National Cancer Database (NCDB) 2013 participant user file was the source of all analyzed data. The NCDB is a prospectively maintained cancer outcomes database that catalogs information from over 70% of new cancer diagnoses annually from more than 1,500 Commission-on-Cancer accredited hospitals across the United States (10). This study was deemed exempt from the requirement of informed consent by the Institutional Review Board of the University of Tennessee Health Science Center given the deidentified nature of all data.
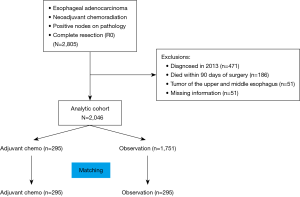
We queried the NCDB for all patients with esophageal adenocarcinoma from 2006 to 2012 who received neoadjuvant chemoradiation therapy and underwent a complete resection but were found to have lymph node metastases on pathologic examination. Patients were excluded if they had non-adenocarcinoma histology, underwent an incomplete resection, or had pathologic N0 disease. Patients with missing information for variables used during propensity matching and patients who died within 90 days of surgery were also excluded from the final analysis. We excluded patients who died within 90 days to decrease selection bias, because patients not able to survive 90 days were unlikely to be candidates for adjuvant therapy. Comparisons were made based on administration of adjuvant chemotherapy as determined by the systemic therapy-surgery sequencing variable.
Propensity matching and statistical analysis
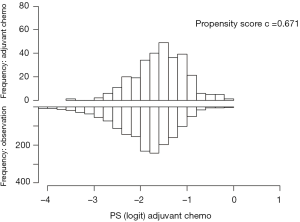
We performed propensity matching to create balanced cohorts of patients who received chemoradiation therapy and had complete resection based on whether they received adjuvant chemotherapy or not. We applied a logistic regression model that generated scores based on the following variables: age, sex, race, Charleson-Deyo comorbidity score, insurance status, treatment facility type, year of diagnosis, tumor grade, tumor size, number of positive lymph nodes on pathologic examination, number of lymph nodes pathologically examined, and American Joint Committee on Cancer (AJCC) T status. The AJCC (7th edition) T status was determined based on the depth of invasion or extension of the tumor as described based on tumor pathology in the NCDB. We used the recommended caliper width of 0.2 times the standard deviation of the logit propensity score (11), and used standardized differences to compare characteristics before and after the match. A standardized difference <10 indicated acceptable balance (12). Discrimination of the propensity matching was tested with the C-statistic (Figure S1).
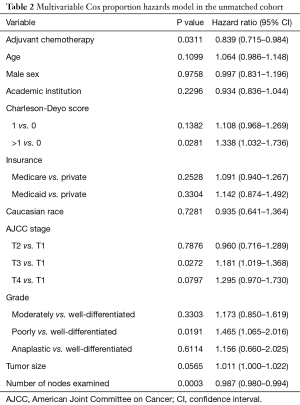
As a sensitivity analysis, we used a multivariable Cox hazards model in the unmatched cohort. The model included the following variables: age, sex, treatment facility type (academic vs. others), Charlson-Deyo comorbidity score, type of insurance, T stage, grade, tumor size, number of nodes examined, and administration of adjuvant chemotherapy.
Patient characteristics are reported using mean ± standard deviation or median and interquartile range (IQR) for continuous variables and as frequencies and percentages for categorical variables. Pearson’s chi square test was used to compare categorical variables, and Student’s t-test was used to compare continuous variables. Survival was analyzed using the Kaplan-Meier method with a log rank analysis, and stratified log-rank in the matched cohort. All survival analyses were performed using overall survival, defined as the time from diagnosis to death or censoring. SAS statistical software package version 9.4 (SAS Institute, Inc., Cary, NC, USA) was used for the analysis. Significance was set at P≤0.05.
Results
Unmatched cohort
We identified 2,805 patients in the NCDB with lymph node metastases detected pathologically after esophagectomy and neoadjuvant chemoradiation therapy for esophageal adenocarcinoma; 2,046 eligible patients were included in the final analysis (Figure 1). Of these, 295 patients received adjuvant chemotherapy, and the remaining 1,751 patients were followed with postsurgical observation. Median follow-up was 1.91 (IQR, 1.17, 3.06) years in the observation group and 2.16 (IQR, 1.36, 3.58) years in the adjuvant chemotherapy group. The median radiation dose received preoperatively by the observation group tended to be higher than in the adjuvant chemotherapy group (5,000 vs. 4,500 rads, P=0.051). The patients in the adjuvant chemotherapy group were significantly younger (57.9 vs. 61.0 years) than the patients in the observation only group and were more likely to be privately insured and male. Patients in the adjuvant chemotherapy group also had significantly more lymph nodes examined, more positive lymph nodes (3.4 vs. 2.8), and less well-differentiated tumors. Patients characteristics were otherwise similar with a standardized difference <10 (Table 1).

Full table
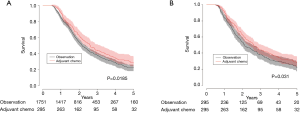
Median overall survival for patients who received adjuvant chemotherapy was 2.6 vs. 2.1 years for patients in the observation only group (31.2 vs. 25.2 months, P=0.0185). The 5-year survival was 27.9% in patients who received adjuvant chemotherapy and 21.5% in patients who did not (Figure 2A). When we used a multivariable Cox proportion hazards model as a sensitivity analysis to identify factors associated with mortality, receipt of adjuvant chemotherapy was associated with improved survival (hazard ratio 0.839, 95% CI: 0.715–0.984, P=0.0311) (Table 2).
Full table
Matched cohort
Propensity matching was performed to create a well-balanced cohort of 295 matched pairs. There were no differences in patient characteristics after propensity matching (Table 1). Median overall survival was 2.6 years in the patients who received adjuvant chemotherapy vs. 2.0 years (31.2 vs. 24.0 months) in patients who were observed until progression (P=0.031). The 5-year survival was 27.9% in the adjuvant chemotherapy group vs. 20.2% in the observation group (Figure 2B), which was consistent with the effect seen in the multivariate analysis in the unmatched cohort.
Discussion
In a large population-based study, we found that adjuvant chemotherapy was associated with improved survival when compared with observation only in patients who received preoperative chemoradiation and were found to have nodal metastases after complete resection. These findings may support consideration for administering adjuvant therapy in patients with nodal metastases found during resection of esophageal adenocarcinoma and may assist in clarifying current guidelines. The findings should also encourage randomized trials addressing the usefulness of adjuvant chemotherapy in patients with nodal metastases after chemoradiation and esophagectomy.
The role of adjuvant chemotherapy in patients with esophageal cancer treated with preoperative chemoradiation has been particularly unclear. In patients who are not treated with neoadjuvant therapy, current guidelines are based on randomized studies in which a minority of patients had esophageal or gastroesophageal (GE) junction adenocarcinoma. An early, multicenter, phase II trial (13) evaluated adjuvant paclitaxel and cisplatin after complete resection in the absence of neoadjuvant therapy. The majority of patients had T3N1 disease (65%, 36/55), and 84% completed four cycles of adjuvant therapy. Median survival was 2.6 years, mirroring the results of our current study and compared favorably with historic controls. Smalley (14) reported on Intergroup Study 0116 in which patients with gastric and GE junction tumors found to be node positive after resection were treated with adjuvant chemoradiation therapy or observation. Only 20% of patients had cancer of the GE junction, and only 65% of patients assigned to adjuvant chemoradiation completed therapy. Nonetheless, patients treated with postoperative chemoradiation had a significant survival advantage. The CLASSIC study of adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy is also cited as supporting evidence for adjuvant chemotherapy in patients with cancers of the digestive tract (15). Although the patients who received adjuvant therapy in the CLASSIC study had better survival than patients who did not, none had esophageal or GE junction tumors.
The available evidence supporting adjuvant chemotherapy in patients who received preoperative therapy is scant. The MRC Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial (16) included 250 patients with gastric or esophageal adenocarcinoma who were treated with 3 preoperative and 3 postoperative cycles of epirubicin, cisplatin and 5-flurouracil and compared their outcomes with 253 patients treated with surgery alone. A significant increase in 5-year overall survival, from 23% in the surgery only group to 36.3% in the chemotherapy group, was seen. The majority of patients who started chemotherapy completed the preoperative course (90.7%), but only 41% of patients completed both the preoperative courses and the postoperative courses, highlighting the difficulty in administering adjuvant treatment after major surgery. Unfortunately, only 25% of patients enrolled in the MAGIC study had esophageal or GE junction cancers, and the rest had gastric cancer. Ychou and colleagues (9) reported a phase III trial comparing preoperative and postoperative chemotherapy with observation alone in patients with adenocarcinoma of the lower esophagus. Midtrial, the inclusion criteria were broadened to include gastric cancers, and ultimately 25% of the patients enrolled had gastric cancer. Only 50% of patients who had at least one cycle of preoperative treatment received postoperative chemotherapy. Survival was significantly improved in patients who received chemotherapy, but the effects of postoperative treatment were not isolated. Additionally, the rate of R0 resection in the surgery only group was relatively low at 74%. A single-institution, retrospective, propensity-matched study (7) compared preoperative chemoradiation with postoperative chemotherapy in patients with stage II or higher esophageal adenocarcinoma. There were no differences in overall or disease-free survival between the two groups. Another retrospective study on preoperative chemotherapy followed by surgery and adjuvant chemotherapy showed improved survival in patients receiving adjuvant chemotherapy (17). Like the MAGIC study, patients with gastric cancers were included, and neoadjuvant radiation was not used.
The overall survival in our cohort is similar to other series of esophageal cancer patients with residual nodal disease. Stiles et al. (18) retrospectively analyzed a cohort of patients treated with neoadjuvant therapy followed by three field en-bloc esophagectomy. Five-year survival for patients with stage III disease was 24%. Speicher et al. (19) noted a 5-year survival of 24% in patients with node positive esophageal cancer who received adjuvant therapy vs. 14% in patients who did not.
The NCDB has been previously used to attempt to clarify the role of adjuvant chemotherapy in patients with esophageal cancer who received neoadjuvant chemoradiotherapy (20). Burt and colleagues found that adjuvant chemotherapy was associated with improved survival only in patients with positive nodes after neoadjuvant therapy, a conclusion concordant with our findings. The previous study differed from ours in that it included patients who had incomplete resections, patients who had negative nodes on the final pathology, and patients with squamous cell cancers of the esophagus. Our present work focused solely on patients with adenocarcinoma who had positive nodes after neoadjuvant chemoradiation therapy and complete surgical resection, delineating the potential benefits of adjuvant chemoradiation in this specific patient population. Additionally, Burt and colleagues used multivariable analysis, while we created two propensity-matched cohorts of similar patients who received adjuvant chemotherapy or not.
The limitations of our study are based on its retrospective design and the absence of certain data points in the NCDB. There are important fields missing from the NCDB including the type of esophagectomy performed, preoperative performance status, postoperative complications, chemotherapy and radiation toxicity, and type of surgery performed. The NCDB also does not allow for disease-free survival calculation. There are no fields that describe grading of the response to neoadjuvant therapy. Additionally, it is not possible to verify the accuracy of the information entered in the database, and errors are possible. Staging data can be problematic, because staging was performed according to the AJCC criteria that were current at the time of each patient’s diagnosis, and the AJCC staging criteria underwent major revision in 2010. To improve accuracy, we used the number of positive nodes for the propensity matching and the depth of invasion of the tumor to designate the T stage. As in all retrospective studies, selection bias is possible and even likely. We attempted to mitigate biases by excluding all patients who died within 90 days of surgery and with careful propensity matching. Patients absent in this analysis included patients who died before or within 90 days of surgery, patients with progressive disease precluding resection, patients with complications related to treatment that limited the receipt or completion of planned therapies, and patients with incomplete resection or a pathologic complete response to neoadjuvant therapy. There is also no way to know if every patient completed his or her planned course of therapy, nor is there information on the type of chemotherapy administered.
In conclusion, adjuvant chemotherapy, when given to patients with persistent nodal metastases after neoadjuvant chemoradiation and complete resection, was associated with improved survival as compared with observation alone. Randomized controlled trials are necessary to further evaluate the role of adjuvant chemotherapy, but until the data from such trials becomes available, consideration should be given to the use of adjuvant chemotherapy in patients with nodal metastases after neoadjuvant therapy and complete resection of esophageal adenocarcinoma.
Acknowledgments
The authors would like to acknowledge Kevin Kennedy, MS for expert biostatistical support, and Shannon Wyszomierski, PhD for expert scientific editorial review.
Footnote
Conflicts of Interest: Presented at the 98th meeting of the American Association for Thoracic Surgery, April 28th–May 1st, 2018, San Diego, CA, USA. B Weksler: Proctor for Intuitive Surgery, speaker for AstraZeneca.
Ethical Statement: This study was approved by the Institutional Review Board of the University of Tennessee Health Science Center who waived the requirement of informed consent given the deidentified nature of all data.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Malhotra GK, Yanala U, Ravipati A, et al. Global trends in esophageal cancer. J Surg Oncol 2017;115:564-79. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- NCCN Guidelines Version 4.2017, Esophageal and Esophagogastric junction cancers. . 2017. Available online: www.nccn.org
- Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016;16:503. [Crossref] [PubMed]
- Zahoor H, Luketich JD, Levy RM, et al. A propensity-matched analysis comparing survival after primary minimally invasive esophagectomy followed by adjuvant therapy to neoadjuvant therapy for esophagogastric adenocarcinoma. J Thorac Cardiovasc Surg 2015;149:538-47. [Crossref] [PubMed]
- NCCN Guidelines Version 1.2018 Esophageal and Esophagogastric Junction Cancers. . 2018. Available online: www.nccn.org
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Armanios M, Xu R, Forastiere AA, et al. Adjuvant Chemotherapy for Resected Adenocarcinoma of the Esophagus, Gastro-Esophageal Junction, and Cardia: Phase II Trial (E8296) of the Eastern Cooperative Oncology Group. J Clin Oncol 2004;22:4495-9. [Crossref] [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389-96. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Saunders JH, Bowman CR, Reece-Smith AM, et al. The role of adjuvant platinum-based chemotherapy in esophagogastric cancer patients who received neoadjuvant chemotherapy prior to definitive surgery. J Surg Oncol 2017;115:821-9. [Crossref] [PubMed]
- Stiles BM, Christos P, Port JL, et al. Predictors of survival in patients with persistent nodal metastases after preoperative chemotherapy for esophageal cancer. J Thorac Cardiovasc Surg 2010;139:387-94. [Crossref] [PubMed]
- Speicher PJ, Englum BR, Ganapathi AM, et al. Adjuvant Chemotherapy Is Associated with Improved Survival after Esophagectomy without Induction Therapy for Node-Positive Adenocarcinoma. J Thorac Oncol 2015;10:181-8. [Crossref] [PubMed]
- Burt BM, Groth SS, Sada YH, et al. Utility of Adjuvant Chemotherapy After Neoadjuvant Chemoradiation and Esophagectomy for Esophageal Cancer. Ann Surg 2017;266:297-304. [Crossref] [PubMed]