Questionnaire survey comparing surgery and stereotactic body radiotherapy for lung cancer: lessons from patients with experience of both modalities
Introduction
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer deaths (1). Approximately 25% of patients with lung cancer have early-stage disease and indication for local therapy (2). This rate is likely to increase following screening recommendations (3). Surgical resection with mediastinal lymph node dissection is the standard therapy for operable, early-stage non-small cell lung cancer (NSCLC). However, patients with lung cancer are often elderly and present with comorbid conditions, meaning they are high-risk patients for surgical resection and may hesitate to be treated. Up to 45% of these patients who are offered operative treatment reject surgical treatment (4,5).
Stereotactic body radiotherapy (SBRT) is the standard treatment for medically inoperable patients with early-stage NSCLC (6,7). SBRT has achieved a good local control rate and low incidence of severe toxicities (8-11). Radiation use has increased in recent decades, and more than 25% of patients aged ≥60 years with stage I NSCLC are treated with radiation in the United States (12).
Patients do not make treatment decisions solely based on reliable evidence of outcomes, but also consider their personal preferences and their clinicians’ recommendations (13). Patient decision-making aids have been successful in informing, involving, and empowering patients to participate in decision-making, particularly in decisions regarding cancer treatment (14,15). Recent guidelines from the American Society of Radiation Oncology (16) and the American Society of Clinical Oncology (17) place increasing emphasis on shared decision-making. The shared information includes treatment choices, probability of outcomes, toxicities, morbidity, mortality, and QOL, along with the patient’s values and preferences (18). In addition, experience-based views of patients who have experienced both surgery and SBRT for lung cancer are thought to be useful for patients facing treatment decisions. We conducted a questionnaire survey with patients who had undergone both treatment modalities to investigate patients’ treatment preferences.
Methods
Patients and survey
From the radiotherapy database in our institution, we extracted patients with lung cancer who were treated with SBRT between 2005 and 2017, and who had also been treated with surgery for lung cancer. We obtained consent to participate in this questionnaire survey from these patients by telephone or directly at a follow-up visit. Questionnaires were mailed to patients who consented to participate, and completed questionnaires were returned to our institution by mail. Alternatively, patients could complete the questionnaire at their follow-up visit. This study was approved by the Ofuna Chuo Hospital Review Board (No. 2017-011).
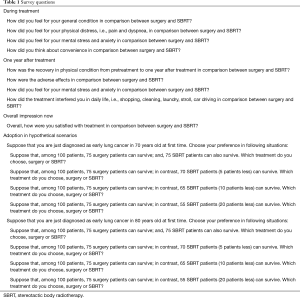
Participating patients were asked questions relating to quality of life (QOL) for both surgery and SBRT, including their perceptions of condition, distress, stress, convenience, adverse effects, satisfaction during and after treatment, and decision-making for hypothetical scenarios (detailed in Table 1 and Figure 1). The questionnaire is our original and is not validated. We instructed patients to circle appropriate numbers on the questionnaire sheet (Figure 1A) with an explanation of the icon arrays (Figure 1B). For explanation of 5-point scale (Figure 1A) in this study, we instructed patients to choose point 5 when they thought SBRT is better definitely, point 4 when SBRT is relatively better, point 3 when they thought surgery and SBRT are equivalent or they could not decide simply, point 2 when surgery is relatively better, point 1 when surgery is better definitely. In questionnaire, we made hypothetical scenarios. In them, we fixed the 5-year overall survival following surgery as 75% and fluctuated those following SBRT: 75% (equivalent), 70% (5% better OS for resection), 65% (10% better OS for resection) 55% (20% better OS for resection).
Full table
Statistical analysis
The frequencies of responses to the scales for each QOL domain were calculated. A one-sample t-test was used to investigate which treatment modality was preferred. An independent t-test was used to investigate factors that affected favorability of treatment modality. A dependent t-test was used to investigate if scales changed for the two situations. For all tests, two-sided P values of <0.05 were considered as statistically significant. Although each scale is a categorical variable, the average score is normally distributed in large samples by the central limit theorem. Thus, we used t-tests for the comparisons in this study. Statistical analyses were conducted by SAS program for Windows, version 9.3 (SAS Institute Inc., Cary, NC, USA) and Microsoft Excel 2013 for Windows.
Results
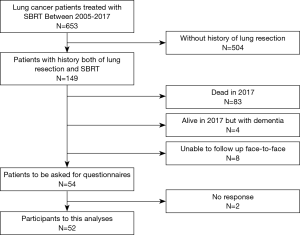
We treated 653 patients with lung cancer with SBRT between 2005 and 2017. Figure 2 presents a flow chart of study participants. Among the 653 patients, 149 also had a history of lung resection. Of these, 83 patients were deceased at the time of this study, four had dementia, and eight were unable to complete follow-up as outpatients in our institution but were followed by a referral medical doctor. Therefore, we invited 54 patients to complete this questionnaire, and received replies from 52 patients.
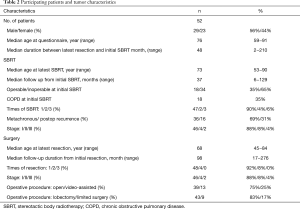
Participating patients and tumor characteristics are listed in Table 2. As for additional SBRT information, treatment time in each SBRT session was approximately 30 minutes. And median number of treatment days was 5 [5–10] days. According to the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0., the number of radiation pneumonitis of grade 2 and 3 were 8 and 1, respectively. Grade 2 rib fracture with intercostal neuralgia was observed in 2 patients. No other toxicities were observed. In total, 48 patients had one resection and four had two resections; 46 patients had SBRT once, four had SBRT twice, and two had SBRT three times. All patients had a history of resection before SBRT. No patients had a history of resection after SBRT. Participants’ median age at the time of the survey was 76 years (range 59–91 years). The median follow-up from initial resection was 98 months (range, 17–276 months), and from SBRT was 37 months (range, 6–129 months). The median duration between latest resection and initial SBRT was 48 months (range, 2–210 months).
Full table
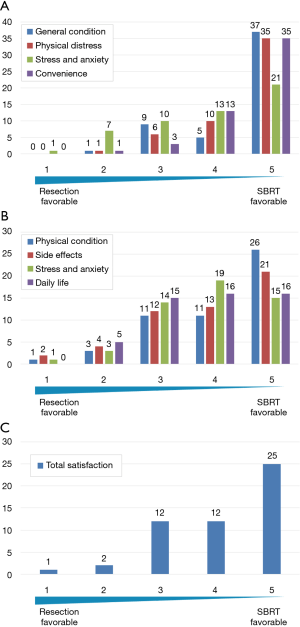
In Figure 3A, each column represents the number of patients for each QOL domain (general condition, physical distress, stress and anxiety, and convenience) during treatment. More patients reported favorable impressions of SBRT in terms of general condition (42 patients, 81%), physical distress (45 patients, 87%), mental stress and anxiety (34 patients, 65%), and convenience (48 patients, 92%). For surgery, only one patient (2%) had a favorable impression of general condition, one (2%) had a favorable impression of physical distress, eight (15%) had a favorable impression of mental stress and anxiety, and one (2%) had a favorable impression of convenience. Similarly, Figure 3B shows that more patients preferred SBRT for physical condition (37 patients, 71%), adverse effects (34 patients, 65%), mental stress and anxiety (34 patients, 65%), and daily life (32 patients, 62%). Fewer patients had more favorable impressions of surgery: physical condition (four patients, 8%), adverse effects (six patients, 12%), mental stress and anxiety (four patients, 8%), and daily life (five patients, 10%). In terms of total satisfaction, 37 patients had a more favorable impression of SBRT and three patients had more favorable impression of surgery (Figure 3C). In addition, patients had a significantly more favorable impression of SBRT during and after treatment, with average scores ranging from 3.8–4.6, by one-sample t-tests against the null values of 3 point scores which meant equivalent value to both of the treatments (all question items; P<0.01). For total satisfaction, older patients (aged ≥76 years) had a significantly less favorable average score (3.8 vs. 4.5) (P=0.02). The other background factors investigated (sex, age, stage at surgery/SBRT, open thoracotomy or video-assisted thoracic surgery, lobectomy or limited surgery, postoperative adjuvant chemotherapy or not, SBRT for postoperative recurrence or metachronous lung cancer, current cancer status, operability or not at initial SBRT, chronic obstructive pulmonary disease or not at initial SBRT) did not significantly affect the score.
In a hypothetical scenario with equivalent treatment outcomes where patients were aged 70 years and faced with decision-making for first-time lung cancer treatment (Figure 4A), the average score was 4.0 under equivalent treatment OS and it showed patients significantly preferred SBRT (P<0.01); 38 (73%) patients preferred SBRT, and only four (8%) patients preferred surgery. Under a scenario of better outcomes for resection, more patients were indecisive, more selected surgery, and average scores significantly declined (3.7, 3.4, and 3.1 for 5%, 10%, and 20% better outcomes for resection, respectively) (P<0.01). However, in a scenario with 20% better outcomes for resection, the preference was statistically insignificant (P=0.47), with 14 (27%) patients preferring SBRT and 12 (23%) preferring surgery.
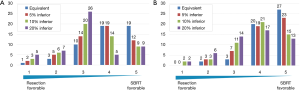
In a scenario for age 80 years, even more patients preferred SBRT compared with the 70-year-old scenario (Figure 4B). Average scores significantly declined for scenarios with 0% (4.4), 5% (4.2), 10% (3.8), and 20% (3.6) better outcomes for surgery (P<0.01). These scores were significantly increased compared with those under the hypothetical scenario involving a 70-year-old patient (P<0.01).
Among the background factors, stage at surgery significantly affected average scores. Patients who were stage II or III at surgery had significantly lower scores than those who were stage I; in patients with stage I, average scores for survival gaps of 0%, 5%, 10%, and 20% (assuming age 70 years) were 3.9, 3.5, 3.2, and 3.0, respectively. In contrast, in patients who were stage II or III, average scores were 4.4, 4.3, 4.1, and 3.6, respectively. No other background factors had a significant effect on score. The 80-year-old scenario showed similar results, with no other background factors significantly affecting the score.
Discussion
Currently, there is no reliable evidence of superiority of surgery or SBRT for patients with operable early-stage NSCLC, especially for high-risk operable patients. Three randomized trials were conducted to compare the outcomes of surgery and SBRT for operable early-stage NSCLC, but these trials closed early because of poor accrual (19). This poor accrual might be explained by the totally different nature of the two treatment modalities (20). In this situation, information regarding patients’ preferences for both modalities is not sufficient. Shaverdian et al. (21) surveyed patient preferences treated with SBRT, and compared them among some part of patients who had experienced both treatment modalities. We also conducted a questionnaire survey with exclusive patients who had experienced both surgery and SBRT for lung cancer and investigated patients’ preferences and treatment decision depending on age. To our knowledge, this is the second study to compare patient preferences among patients who had experienced both treatment modalities.
A major concern for patients when making treatment decisions is QOL during and after treatment. QOL studies following SBRT showed little deterioration in functional ability, reported pulmonary symptoms, and other QOL measures (22-25). However, QOL studies following surgery reported distinct deterioration in physical function and general and pulmonary symptoms (26,27), although these were modestly improved with video-assisted thoracic surgery (28). In a direct comparison using an objective index, QOL measures were significantly more favorable with SBRT (29).
This study was based on a questionnaire survey involving patients who had experienced both surgery and SBRT, an uncommon patient group with valuable insights. Their preferences and QOL may be applicable to early lung cancer patients diagnosed at first time, although the participants themselves were somewhat specific. Participants were treated with SBRT for metachronous second primary lung cancer or postoperative stump recurrence. In this study, the reason SBRT was conducted did not significantly affect the scores, and SBRT was effective for both diseases. The risk of metachronous second primary lung cancer in patients with previously treated lung cancer is thought to be 1.10% per patient per year (30). The rate of postoperative stump recurrence alone is reported to be 3%, even after complete resection (31), and the rate further increased in surgical margin positive patients. To rescue such patients, SBRT plays an important role with high local control (32).
In this study, questions about QOL during and after treatment showed the average scores favored SBRT. These results indicated that patients generally experienced less stress with SBRT, mentally, physically, and comprehensively. A similar result was observed in the previously mentioned study by Shaverdian et al. (21). In addition, both studies showed that the more favorable QOL was consistent even if they had SBRT-related toxicities.
Morbidity and mortality are also major concerns in deciding about treatment, and affect QOL after treatment. This information should also be conveyed to patients, as early death is of particular concern. Post-treatment early mortality rates (0–3 months after treatment) were moderately higher following surgery than SBRT (33). In addition, differences in the rates increased with age (33). In the medium term (3–18 months after treatment), mortality rates were also higher following surgery (34), with excess non-cancer deaths also observed more often in the surgical arm.
In the hypothetical scenarios in the present study, participants generally selected SBRT. In those scenarios, the average score only showed favor of either modality when the difference of the 5-year survival rate of both treatment methods was 20% at the age of 70 years. This result suggests that more patients place importance on better QOL than on good treatment outcomes than physicians may expect. In fact, in the scenario of a 20% difference, the number of patients selecting surgery was small and more than half scored “3”, suggesting that patients could not select a modality.
In terms of the influence of background factors, more advanced stage (stage II/III) at surgery encouraged patients to select SBRT. However, interpretation of this result was difficult because no other factors (including invasiveness of surgery (open vs. VATS, lobectomy vs. limited surgery) and presence of adjuvant chemotherapy) showed significant differences.
Patients’ preferences might be quite different between experiencers and non-experiencers. Tong et al. (13) performed a conjoint analysis using healthy volunteers to reveal patients’ preference in hypothetical stage I NSCLC. The survey respondents were recruited by an open online platform hosted and governed by Amazon, with a self-reported smoking history and age greater than 40 years. They were shown brief characteristics of treatment modalities composed of each numerical values of length of procedure, days in hospital and recovery time at home. Then they answered questions for treatment decision among three treatment options (open operations, minimally invasive operations, and SBRT) with median time of 12 minutes. As results, they preferred minimally invasive operation over SBRT or open operations. Risk of cancer recurrence, as well as treatment modality, were the most important factors associated with treatment preferences. Those results were different from our results. Although many other factors might influence results such as supplied information, characteristics of survey responders (i.e., age, health and comorbidities) and questions, preferences of experiencers are thought to be more persuasive and worth considering for decision making as experience is the best teacher.
Differences in preference and values between patients and physicians may lead to an inappropriate goal in decision-making. As a key part of treatment decision-making, physicians should be aware that their explanations are likely to be biased by their own expertise. It is important to remember that medical specialists disagree on which option is best for patients with early-stage disease. A previous study asked thoracic oncologists to recommend surgery or SBRT for 16 hypothetical patients with stage I NSCLC (35). The results showed limited consistency in the majority of cases, except in the fit of young patients with no comorbidity for surgery. SBRT was recommended more often by pulmonologists and radiation oncologists than by thoracic surgeons. In addition, nearly 55% of thoracic oncologists indicated they considered surgery and SBRT to be equal treatment options. Clinicians who considered surgery and SBRT to be similarly effective were more likely to recommend SBRT.
The present study has several limitations and biases. First, this was a retrospective study performed at a single-institution with a small number of patients, which could not validate the subgroup analyses for any definitive conclusions. Second, all participants were treated with surgery first and SBRT second. There is potential for recall bias and recency bias. Patients might have lost memories of surgery, and evaluate their preferences based on recent memories. Early-death patients were not included in the study sample, which represents potential selection and length bias. Patients were more advanced in age/frailer and thus preferred SBRT based on their current health state. Third, there may be a bias that some patients had a bad impression to surgical operation because they recurred following surgery and were salvaged by SBRT. Fourth, this study was conducted by staff at a radiation oncology center. Participants’ answers might have been different if the study had been conducted by surgeons. Fifth, surgical techniques are progressing and becoming less invasive. With the increase in minimally invasive and robotic surgery procedures, a more contemporaneous cohort would state preferences differently. Sixth, there is no reliable method to compare QOLs after one treatment to those after another. Therefore, we performed this questionnaire with our original method though it is not evaluated objectively. In order to exclude those biases, it would be desirable to prospectively conduct this questionnaire survey closer to the decision time and at fixed times after each treatment with larger population.
The experience-based views of patients who had experienced both surgery and SBRT for lung cancer are valuable and helpful to both physicians and patients in treatment decision-making. Physicians should be aware of and respect these valuable experience-based views, and make an effort to share them with their patients. Patients should be aware of such information to aid discussion and reaching appropriate treatment decisions with their physician. In conclusion, most patients who had experienced both surgery and SBRT for lung cancer prefer SBRT. This information would be helpful to patients and physicians in treatment decision-making.
Acknowledgments
We thank Audrey Holmes, MA, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. Dr. Takeda reports a Varian Research Grant and a grant from the Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science when conducting this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ofuna Chuo Hospital Review Board (No. 2017-011). We obtained consent to participate in this questionnaire survey from these patients by telephone or directly at a follow-up visit.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Moyer VA, Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA 2010;303:2368-76. [Crossref] [PubMed]
- Ryoo JJ, Ordin DL, Antonio AL, et al. Patient preference and contraindications in measuring quality of care: what do administrative data miss? J Clin Oncol 2013;31:2716-23. [Crossref] [PubMed]
- Boily G, Filion E, Rakovich G, et al. Stereotactic Ablative Radiation Therapy for the Treatment of Early-stage Non-Small-Cell Lung Cancer: CEPO Review and Recommendations. J Thorac Oncol 2015;10:872-82. [Crossref] [PubMed]
- Vansteenkiste J, Crino L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Guckenberger M, Allgauer M, Appold S, et al. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol 2013;8:1050-8. [Crossref] [PubMed]
- Ricardi U, Frezza G, Filippi AR, et al. Stereotactic Ablative Radiotherapy for stage I histologically proven non-small cell lung cancer: an Italian multicenter observational study. Lung Cancer 2014;84:248-53. [Crossref] [PubMed]
- Dalwadi SM, Szeja SS, Bernicker EH, et al. Practice Patterns and Outcomes in Elderly Stage I Non-Small-cell Lung Cancer: A 2004 to 2012 SEER Analysis. Clin Lung Cancer 2018;19:e269-76. [Crossref] [PubMed]
- Tong BC, Wallace S, Hartwig MG, et al. Patient Preferences in Treatment Choices for Early-Stage Lung Cancer. Ann Thorac Surg 2016;102:1837-44. [Crossref] [PubMed]
- O'Brien MA, Whelan TJ, Villasis-Keever M, et al. Are cancer-related decision aids effective? A systematic review and meta-analysis. J Clin Oncol 2009;27:974-85. [Crossref] [PubMed]
- Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. [PubMed]
- Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2018;36:710-9. [Crossref] [PubMed]
- Hammermeister KE, Henderson WG, Bronsert MR, et al. Bringing Quantitative Risk Assessment Closer to the Patient and Surgeon: A Novel Approach to Improve Outcomes. Ann Surg 2016;263:1039-41. [Crossref] [PubMed]
- Louie AV, Palma DA, Dahele M, et al. Management of early-stage non-small cell lung cancer using stereotactic ablative radiotherapy: controversies, insights, and changing horizons. Radiother Oncol 2015;114:138-47. [Crossref] [PubMed]
- Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol 2014;32:2847-54. [Crossref] [PubMed]
- Shaverdian N, Wang PC, Steinberg M, et al. The patient's perspective on stereotactic body radiation therapy (SBRT) vs. surgery for treatment of early stage non-small cell lung cancer (NSCLC). Lung Cancer 2015;90:230-3. [Crossref] [PubMed]
- Sun V, Kim JY, Williams AC, et al. Quality of life and symptoms following stereotactic body radiotherapy in early-stage lung cancer patients. J Community Support Oncol 2014;12:407-14. [Crossref] [PubMed]
- Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol 2012;7:1148-54. [Crossref] [PubMed]
- van der Voort van Zyp NC, Prevost JB, van der Holt B, et al. Quality of life after stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2010;77:31-7. [Crossref] [PubMed]
- Videtic GM, Reddy CA, Sorenson L. A prospective study of quality of life including fatigue and pulmonary function after stereotactic body radiotherapy for medically inoperable early-stage lung cancer. Support Care Cancer 2013;21:211-8. [Crossref] [PubMed]
- Kenny PM, King MT, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J Clin Oncol 2008;26:233-41. [Crossref] [PubMed]
- Poghosyan H, Sheldon LK, Leveille SG, et al. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer 2013;81:11-26. [Crossref] [PubMed]
- Gazala S, Pelletier JS, Storie D, et al. A systematic review and meta-analysis to assess patient-reported outcomes after lung cancer surgery. ScientificWorldJournal 2013;2013:789625. [Crossref] [PubMed]
- Louie AV, van Werkhoven E, Chen H, et al. Patient reported outcomes following stereotactic ablative radiotherapy or surgery for stage IA non-small-cell lung cancer: Results from the ROSEL multicenter randomized trial. Radiother Oncol 2015;117:44-8. [Crossref] [PubMed]
- Thakur MK, Ruterbusch JJ, Schwartz AG, et al. Risk of Second Lung Cancer in Patients with Previously Treated Lung Cancer: Analysis of Surveillance, Epidemiology, and End Results (SEER) Data. J Thorac Oncol 2018;13:46-53. [Crossref] [PubMed]
- Lopez Guerra JL, Gomez DR, Lin SH, et al. Risk factors for local and regional recurrence in patients with resected N0-N1 non-small-cell lung cancer, with implications for patient selection for adjuvant radiation therapy. Ann Oncol 2013;24:67-74. [Crossref] [PubMed]
- Takeda A, Sanuki N, Eriguchi T, et al. Salvage stereotactic ablative irradiation for isolated postsurgical local recurrence of lung cancer. Ann Thorac Surg 2013;96:1776-82. [Crossref] [PubMed]
- Stokes WA, Bronsert MR, Meguid RA, et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:642-51. [Crossref] [PubMed]
- Rusthoven CG, Palma DA, Senan S, et al. The Head Start Effect: Will Acute and Delayed Postoperative Mortality Lead to Improved Survival with Stereotactic Body Radiation Therapy for Operable Stage I Non-Small-Cell Lung Cancer? J Clin Oncol 2017;35:1749-51. [Crossref] [PubMed]
- Hopmans W, Zwaan L, Senan S, et al. Differences between pulmonologists, thoracic surgeons and radiation oncologists in deciding on the treatment of stage I non-small cell lung cancer: A binary choice experiment. Radiother Oncol 2015;115:361-6. [Crossref] [PubMed]