
Learning curve for robot-assisted lobectomy of lung cancer
Introduction
Robot-assisted thoracoscopic surgery (RATS) is a minimally invasive lung surgery method for early stage non-small cell lung cancer (NSCLC). Since the first report on robot-assisted lobectomy which was performed using a da Vinci surgical system in 2002 (1), utility incision assisted robotic lobectomy, performed with one utility incision and several ports, has been widely used for lung surgery (2-4). Park et al. (5) and Veronesi et al. (6) described their experience in robot-assisted lobectomy with two thoracoscopic ports and a 4-cm utility incision in 2006 and 2011, respectively. In addition, robotic lobectomy has been reported to be a feasible and safe approach (3,7,8), with clinical outcomes similar to those of open and video-assisted thoracoscopic surgery (VATS) (9,10). However, the da Vinci system is still not covered by the national health insurance of China, and it has only been used in few patients. Up to now, few studies have systematically analyzed the learning curve of robotic lobectomy.
Initially, the cumulative sum (CUSUM) technique was successfully used to monitor the trend of continuous variation in the industrial sector, and it was later adopted in medicine to analyze the learning curve in the 1970s (11,12). It has already been used in several types of surgical processes, including laparoscopic surgery (13,14), robotic laparoscopic surgery (15,16), and esophagectomy (17,18). Our purpose is to define the learning curve to provide training guideline of RATS lobectomy by reporting our experience using the CUSUM technique.
Methods
Study population
Lung cancer patients who underwent robot-assisted lung surgery by a single team in the Department of Thoracic Surgery of the Affiliated Hospital of Qingdao University between October 2014 and October 2016 were included in this study. All patients who underwent R0 resection and radical lymph node dissection were pathologically confirmed as having NSCLC. Patients under preoperative chemoradiotherapy were excluded. A total of 208 patients were ultimately enrolled. Informed consent for robotic assistance was obtained from all patients, and all surgeries were performed constantly during the study period. This study was approved by the institutional review board of our center.
The data collected included age, sex, FEV1, ECOG performance status, tumor size, histopathological results, operative procedure, tumor site, clinical TNM stage, operative time, intraoperative estimated blood loss, conversion rates to open surgery, length of postoperative hospital stay, chest tube duration, perioperative complications, total number of dissected lymph nodes (LNs), and number of dissected LN stations. Definitions of operation duration are as followed: (I) docking time, the time from creation of portal incisions to the end of the docking; (II) surgeon console time, the time the surgeon spent at the console performing operations; (III) total surgical time, from the start of incision open to the end of closure (skin to skin).
Surgical technique
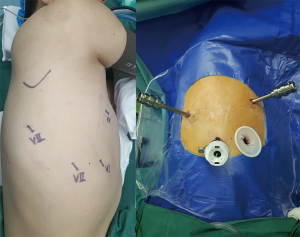
All robotic lung surgeries were performed with da Vinci Si surgical robotic system (Intuitive Surgical, Inc., Santa Clara, CA, USA). Patients received general anesthesia with double-lumen endotracheal intubation to achieve single-lung ventilation. Three ports and a utility incision were used in the robotic assisted lobectomy using 3 arms of the system (RAL-3) (19): a 12-mm camera port in the 7th intercostal space (ICS) at the midaxillary line and two 8-mm working ports in the 4th ICS at the anterior axillary line and the 7th ICS at the posterior axillary line. A 2.5-cm auxiliary incision was finally created in the 6th ICS without rib spreading, using an applicable wound protector (Figure 1). Specimen were obtained through the auxiliary incision at the end of the procedure.
All surgeries were carried out by the same surgeon, who had already performed more than 500 conventional video-assisted pulmonary resections before the RATS technique was adopted in our center.
Statistical analysis
The cumulative summation (CUSUM) which was calculated on the basis of the mean operative time, was used to analyze the learning curves of RATS lobectomy. Firstly, the cases were arranged in chronological order. Next, the CUSUM of the first case was the difference between the value for the first case and the mean for all cases. The CUSUM of the second case was the CUSUM of first case added to the difference between the second case and the mean for all cases. This recursive process continued until CUSUM for the last case was calculated as zero (15,17). A learning curve is deemed to be complete when a decreasing time-point is observed from the CUSUM plot.
SPSS software (v.22.0 for Windows; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Continuous variables are expressed as mean value ± standard deviation, and categorical variables are expressed as percentages. We performed a comparison among the three groups, in which the independent sample and paired t-test were used for continuous variables. The Fisher exact test was applied for dichotomous variables. P<0.05 was considered a significant difference. Learning curves were analyzed with MATLAB 2014.
Results
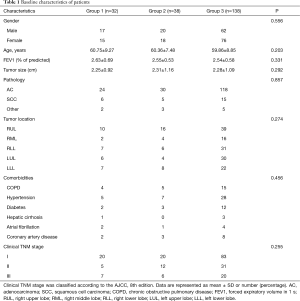
A total of 252 patients who underwent RATS lung resection were included in this study. Among the included patients, 44 patients who received robotic assisted wedge resection or segmentectomy were excluded, 208 patients who received robot assisted lobectomy were finally enrolled. None of the patients was under preoperative neoadjuvant therapy. The study participants consisted of 99 men (47.6%) and 109 women (52.4%), with an average age of 60.09 years. Baseline characteristics of patients are summarized in Table 1. No significant differences were found in gender, age, preoperative comorbidities and FEV1%, histology, clinical stage among the groups.
Full table
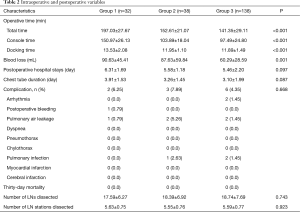
According to the cross point of the cumulative plots, the first 32 patients who underwent robotic lobectomy were assigned to Group 1, and the other 33rd to 70th patients were assigned to Group 2, while the remaining 71st to 208th cases were assigned to Group 3. We analyzed the learning curve for surgical time using the CUSUM method and found that a decreasing point for total surgical time begun at the 32nd operation (Figure 2A). Meanwhile, a similar trend at the 34th operation was observed for surgeon console time by visually inspecting the CUSUM plots (Figure 2B). Docking time decreased at the 20th operation (Figure 2C). Among the three groups, the surgical time (197.03±27.67, 152.61±21.07, 141.35±29.11 min, P<0.001), console time (150.97±26.13, 103.89±18.04, 97.49±24.80 min, P<0.001) and docking time (13.53±2.08, 11.95±1.10, 11.89±1.49 min, P<0.001) were decreased. There was no case of conversion to VATS or open thoracotomy in the 208 consecutive cases.

Additionally, multiple intraoperative variables and short-term postoperative outcomes were compared as shown in Table 2. The number of dissected LNs (17.59±6.27 vs. 18.39±6.92 vs. 18.74±7.69, P=0.743) and dissected LN stations (5.63±0.75 vs. 5.55±0.76 vs. 5.59±0.77, P=0.923) were not significantly different among the groups. However, compared with Group 1, Group 2 and Group 3 displayed low blood loss (90.63±45.41, 87.63±59.84, 60.29±28.59 mL, P=0.001) and had shorter operative time (197.03±27.67, 152.61±21.07, 141.35±29.11 min, P<0.001). Drainage duration (3.91±1.53, 3.26±1.45, 3.10±1.99 d, P=0.087) and length of postoperative stay (6.31±1.69, 5.58±1.18, 5.46±2.20 d, P=0.097) were not different among the three groups. The postoperative complications and the incidence rate for each cohort were also assessed. There was no 30-day mortality in any cohort.
Full table
Discussion
Previous studies have shown several advantages of RATS lobectomy and demonstrated good short-time outcomes, including a reduction in the length of hospital stay and generally few post-operative complications (20,21). In the present study, none of the 208 consecutive patients who underwent robotic lobectomy was converted to open thoracotomy. In addition, although a significant decrease in the surgical time was noted after the first 32 cases, the length of hospital stay, estimated blood loss, conversion rate, and the frequency of major comorbidities were not significantly different among the three groups. Furthermore, there was no 30-day mortality in any group, which suggests that RATS lobectomy is safe and feasible.
At the initial stage of applying the robotic system, low availability and high costs may render surgeons unable to gain sufficient learning experience. Therefore, some of the initial operations are considered as the learning phase for the surgeon. By analyzing the learning curve, a novice surgeon can train and evaluate the benefits of the robotic lobectomy.
According to the CUSUM plots, we observed that the cutoff point of the surgical time learning curve of robotic lobectomy was the 32nd case, which implies that 32 cases are needed for surgeons who are experienced in open thoracotomy and VATS to achieve early proficiency. However, in a report comprising 185 patients treated with robotic lobectomy using 3 arms, the learning curve based on operative times, mortality and surgeon comfort was 15, 20 and 19 cases, respectively according to the slope of the curve corresponding to the beginning of the plateau (22). Veronesi suggested that 18 cases may be the learning curve for robotic lobectomy using a four-armed robotic way, based on decreased operating time trends in 54 lung cancer patients (23). Baldonado et al. (24) argued that experienced surgeons in VATS may not have a definite learning curve for robotic lobectomy.
By making comparisons among these studies, our results can be interpreted as follows. Firstly, this study evaluates the in the initial stage of the learning curve of a single surgeon and his team in 208 consecutive cases, using the CUSUM technique which is a more accurate statistical method (25). The learning curve may change from surgeon to surgeon. Additionally, we focus on surgical time in the analysis since it is the most widely used marker for the learning curve in this report. We speculate that the learning period of 32 operations is due to the difficulties associated with communication during the surgical procedure, since the surgeon sits away from the bed implying that the assistant must help the surgeon through the utility incision to expose the hilum structure. Nevertheless, a surgeon with an extensive VATS lobectomy experience and familiarity with the thoracoscopic appearance of pulmonary anatomy will have a short learning curve of the surgical procedure and a low rate of conversion.
The phase I, phase II and phase III periods were the 1st–32nd, 33rd–70th and 70th–208th operations, respectively. Phase I was the initial learning period, in which the CUSUM of the operative time increased. Phase II was the consolidation period, in which the operative time reached a mean value and remained stable. At the beginning of this phase, the operator was considered to have reached the learning point. This result suggested that the surgeon attained deeper understanding of anatomy and better collaboration with the teammates. Phase III was the experienced period in which CUSUM decreased continuously without increased morbidity or mortality in the cohort. This may explain the decreased estimated blood loss after the different phases. In phase I of the initial learning period, multiple factors such as the stress of role of the surgeon (primary operator or assistant) and the skilled degree may influence the cooperation and exposure of anatomical structure with increased risk of vascular injury. After the learning period, better understanding and cooperation result in less intraoperative injuries. The whole surgical time contains console time that requires cooperation between the surgeon and the bedside assistant, and docking time that requires incision design, portal set-up, docking, undocking and changing instruments. Therefore, the proficiency of the bedside assistant is crucial in the entire operation process. After 20 cases, the docking time decreased and it was significantly different among the groups (13.53±2.08, 11.95±1.10, 11.89±1.49, P<0.001), which implies that the learning curve for a skilled bedside assistant requires 20 cases.
However, there are several limitations in this study. Since it is a retrospective study design that evaluated the learning curve of a single surgeon and his team. Other parameters including tube drainage duration, length of hospital stay and long-term oncology outcomes should be analyzed in future prospective multicenter studies involving several surgeons. In conclusion, robotic lobectomy is safe and feasible for patients with lung cancer based on its short-term outcomes. For a surgeon who is experienced in open thoracotomy and VATS, 32 operations are needed to attain early proficiency.
Acknowledgments
Funding: This work was supported by Qingdao City: Science and technology for People’s Livelihood (Grand NO. 17-3-3-5-nsh).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol for data collection was approved by the Institutional Review Board of the Affiliated Hospital of Qingdao University, and informed consent was waived.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Rajaram R, Mohanty S, Bentrem DJ, et al. Nationwide Assessment of Robotic Lobectomy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;103:1092-100. [Crossref] [PubMed]
- Huang J, Luo Q, Tan Q, et al. Initial experience of robot-assisted thoracoscopic surgery in China. Int J Med Robot 2014;10:404-9. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Robotic Lobectomy: Review of a National Database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: Technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Veronesi G, Agoglia BG, Melfi F, et al. Experience with robotic lobectomy for lung cancer. Innovations (Phila) 2011;6:355-60. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B. Robot-Assisted Thoracoscopic Lobectomy for Early-Stage Lung Cancer. Ann Thorac Surg 2008;85:1880-5; discussion 1885-6.
- Adams RD, Bolton WD, Stephenson JE, et al. Initial multicenter community robotic lobectomy experience: Comparisons to a national database. Ann Thorac Surg 2014;97:1893-8; discussion 1899-900.
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Wohl H. The Cusum Plot: Its Utility in the Analysis of Clinical Data. N Engl J Med 1977;296:1044-5. [Crossref] [PubMed]
- Chaput de Saintonge DM, Vere DW. Why don't doctors use cusums? Lancet 1974;1:120-1. [Crossref] [PubMed]
- Okrainec A, Ferri LE, Feldman LS, et al. Defining the learning curve in laparoscopic paraesophageal hernia repair: A CUSUM analysis. Surg Endosc 2011;25:1083-7. [Crossref] [PubMed]
- Tekkis PP, Senagore AJ, Delaney CP, et al. Evaluation of the learning curve in laparoscopic colorectal surgery: Comparison of right-sided and left-sided resections. Ann Surg 2005;242:83-91. [Crossref] [PubMed]
- Bokhari MB, Patel CB, Ramos-Valadez DI, et al. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 2011;25:855-60. [Crossref] [PubMed]
- Buchs NC, Pugin F, Bucher P, et al. Learning curve for robot-assisted Roux-en-Y gastric bypass. Surg Endosc 2012;26:1116-21. [Crossref] [PubMed]
- Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: Description of a learning curve. J Am Coll Surg 2014;218:1130-40. [Crossref] [PubMed]
- Zhang H, Chen L, Wang Z, et al. The Learning Curve For Robotic McKeown Esophagectomy In Patients With Esophageal Cancer. Ann Thorac Surg 2018;105:1024-30. [Crossref] [PubMed]
- Cerfolio R, Louie BE, Farivar AS, et al. Consensus statement on definitions and nomenclature for robotic thoracic surgery. J Thorac Cardiovasc Surg 2017;154:1065-9. [Crossref] [PubMed]
- Farivar AS, Cerfolio RJ, Vallières E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the society of thoracic surgeons database. Innovations (Phila) 2014;9:10-5. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Baldonado JJAR, Amaral M, Garrett J, et al. Credentialing for robotic lobectomy: what is the learning curve? A retrospective analysis of 272 consecutive cases by a single surgeon. J Robot Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Liu X, Chen X, Shen Y, et al. Learning curve for uniportal video-assisted thoracoscopic surgery lobectomy—results from 120 consecutive patients. J Thorac Dis 2018;10:5100-7. [Crossref] [PubMed]


