Lobectomy in octogenarians: real world outcomes for robotic-assisted, video-assisted thoracoscopic, and open approaches
Introduction
Over the past few decades, it has been increasingly recognized that the aging population will require surgeons to care for older and frailer patients than in the past. By 2020, there will be an estimated 51% increase in oncologic procedures in the elderly compared to the previous decade (1). Particular interest has focused on the octogenarian population, which is one of the fastest growing segments of the elderly (2). With individuals living to progressively higher chronological ages along with the surge in the baby boomer generation, it is necessary to re-evaluate assumptions about oncologic care for the elderly. Today, an octogenarian man has a life expectancy of 5–9 years and an octogenarian woman has a life expectancy of 5–10 years (3). In this context, a diagnosis of early stage lung cancer in an octogenarian should be approached with curative intent, barring any prohibitive risk factors.
Lobectomy remains the standard of care for surgical treatment of invasive non-small cell lung cancer (NSCLC); in the octogenarian population, lobectomy is feasible and associated with acceptable outcomes. Although nearly all published reports on this subject have been retrospective with small sample sizes and subject to selection bias, many series show cancer-specific survival that is equivalent to that in younger patients (2,4). Nevertheless, in the immediate postoperative period, octogenarians experience increased complications and mortality compared to younger patient populations (4). This concern has led to reluctance to offer surgery and increasing interest in alternatives such as stereotactic body radiation therapy (SBRT). For example, in the Netherlands, the rate of SBRT for patients >75 years old increased from 26% to 42% in less than a decade (5). Moreover, the increasing number of published claims about the superiority of SBRT over surgery even in operable patients raises questions about the role of surgery (6).
A minimally invasive approach for the octogenarian lung cancer patient has been advocated for improved postoperative outcomes in this higher risk patient population (7-9). These early reports focused on video-assisted thoracoscopic surgery (VATS), but more recently robotic-assisted lobectomy has been considered an alternative minimally invasive technique with rapid adoption. As of 2015, robotic-assisted lobectomy in the United States comprised nearly 20% of all elective lobectomies as reported in the Premier database (10). In light of this shift, we sought to investigate the role of all minimally invasive techniques in the octogenarian population by evaluating the perioperative outcomes of standard open lobectomy to both VATS and robotic-assisted lobectomy using a nationwide administrative database. We hypothesized that both minimally invasive techniques would be associated with improved outcomes over open thoracotomy in this frail patient group.
Methods
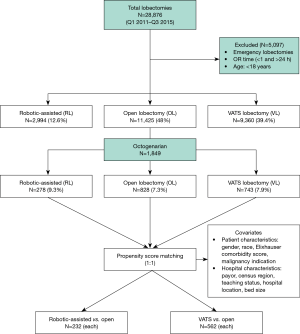
A retrospective database study was conducted using the Premier Healthcare Database, which is a large, U.S. hospital-based, all-payer and service-level database. The Premier database provides information from approximately 20% of the total inpatient hospital discharges in the United States. It includes data from geographically diverse populations in both rural and urban communities. Both academic and community hospitals are represented. The database contains aggregated, de-identified patient information, and is HIPAA-compliant; therefore, no institutional review board approval was required for this study [45 CFR 46.101(b)(4)]. Patients who were 80 years of age or older and who underwent elective lobectomy by robotic-assisted, VATS and open approaches from January 1, 2011 through September 30, 2015 were included in the study (Figure 1). Emergency cases and those with an operating room time of less than 1 hour and greater than 24 hours were excluded from the study. The different surgical approaches were identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) procedure codes and current procedure terminology (CPT) codes. The ICD-9-CM codes were also used to identify complications and any conversions to open lobectomy (V64.41 and v64.42) (1). There was a change in the ICD coding system at the end of September 2015 from ICD-9 to ICD-10. To maintain consistency in assessing medical comorbidities and complications, the study period was limited through September of 2015 to avoid confounders associated with ICD-9 to ICD 10 code transition.
Procedures were labelled as conversion to open only when conversion codes were used in association with VATS or robotic-assisted approaches. For the purpose of this analysis, any use of thoracoscopy prior to an intended open thoracotomy was not considered as a conversion to open and have been coded as open procedure. Analyzed data included baseline patient characteristics (age, gender, race, type of malignancy), hospital characteristics (census region, urban or rural, number of beds, teaching status, payor type), perioperative outcomes (operating room time, conversion to open, blood transfusions, length of stay), discharge status, and complications thorough 30 days. The periods at which complications were assessed included intraoperative, postoperative (surgery through discharge), and 30-days (admission through 30 days). Peri-operative morbidity and mortality has been limited to 30-days due to lack of uniform longitudinal data beyond 30-days. Risk stratification of patient comorbidities was performed using the Elixhauser comorbidity index, a validated means of categorizing 31 comorbidities of patients based on ICD-9 codes (11).
One-to-one propensity score matching (PSM) with a caliper size of 0.01 was performed to lessen the potential for selection bias between the surgical approaches (12). Matching was performed using the nearest neighbor method and two separate comparisons were made: robotic-assisted lobectomy (RL) versus open lobectomy (OL) and VATS lobectomy (VL) versus open lobectomy. Covariates included in the matching were: patient characteristics (gender, race, type of malignancy, Elixhauser comorbidity score) and hospital characteristics (payor type, location of the hospital (urban/rural), number of beds in a hospital, census region and teaching status). Univariate analysis was performed before and after PSM to compare the surgical approaches—robotic-assisted, VATS and open—using the Student’s t-test for continuous variables and Chi-squared test or Fisher’s exact test for categorical variables. Two-sided P values <0.05 were considered statistically significant. Sample selection and creation of analytic variables were performed using Instant Health Data (IHD) platform (Boston Health Economics, Inc., Waltham, MA). Statistical analyses were undertaken with R-statistical software, version 3.2.1.
Results
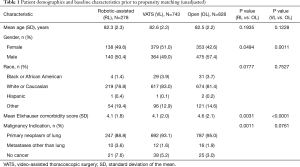
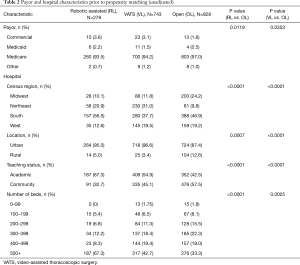
From January 2011 through September 2015 a total of 28,876 lobectomies were reported to the Premier database of which 23,779 lobectomies were elective. A total of 1,849 octogenarian patients met the inclusion criteria. The distribution of surgical approaches consisted of 828 (44.8%) open, 743 (40.1%) VATS, and 278 (15%) robotic-assisted lobectomies. After propensity matching for two comparisons, 87% of patients who underwent open lobectomy in the RL vs. OL comparison arm overlapped with the patients who underwent open lobectomy in the VL vs. OL comparison arm, indicating that a similar open lobectomy population was compared to both RL and VL cohorts. The demographic, payor, and hospital characteristics of unmatched patients are shown in Tables 1,2 and depict statistically significant differences in gender, Elixhauser comorbidity score, type of malignancy, and all hospital characteristics. After propensity-score matching, patient populations were similar across both comparisons.
Full table
Full table
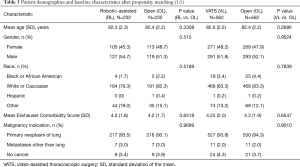
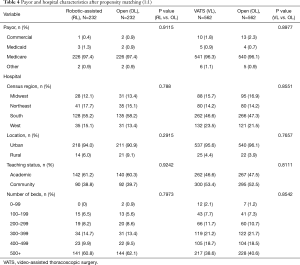
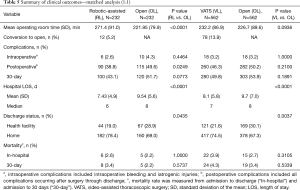
The first comparative analysis was of propensity-matched patients in the open and robotic-assisted lobectomy cohorts (n=232 in each group); patient and hospital characteristics were statistically similar after matching (Tables 3,4). Perioperative outcomes also were compared between matched robotic-assisted and open approaches (Table 5). Mean operating-room time (wheels-in to wheels-out) was significantly longer in the robotic-assisted cohort by 49 minutes, but there was no difference in intraoperative complication rate. There was a 5% rate of conversion to open in the RL cohort. Postoperatively, RL was associated with a significantly lower overall complication rate than the OL cohort (38.8% vs. 49.6%, respectively; P=0.0249) and a shorter length of stay by a median of 2 days (P<0.0001). Significantly more patients in the RL cohort were discharged directly to home (78.4% vs. 69.0%) rather than to an intermediate healthcare facility (18.9% vs. 28.8%, respectively; P=0.0435). Postoperative and 30-day mortality rates were similar between cohorts.
Full table
Full table
Full table
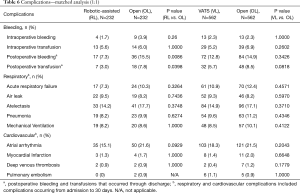
Types of complications (11) are reported in Table 6. At an individual complication level, RL had significantly less post-operative bleeding (7.3% vs. 15.5%, P=0.0086) and need for transfusion (3.0% vs. 7.8%, P=0.0398) compared to OL There were similar rates of acute respiratory failure (7.3% in RL vs. 10.3% in OL; P=0.3264) and postoperative pneumonia (8.2% in RL vs. 9.9% in OL; P=0.6274). There was no difference in air leak between the two cohorts (9.5% in RL vs. 8.2% in OL; P=0.7436). There was a trend toward fewer atrial arrhythmias in the RL cohort, but this did not reach statistical significance (P=0.0929).
Full table
The second analysis was a comparison of propensity-matched patients in the open and VATS lobectomy cohorts (n=562 in each group); the statistically similar patient and hospital characteristics after matching are presented in Tables 3,4. Perioperative outcomes are presented in Table 5. Mean operative time (wheels-in to wheels-out) was slightly longer for VL but the difference did not reach statistical significance. There was a 14% rate of conversion to open in the VL cohort; however, the intraoperative complication rate was similar between cohorts. There was no difference in the overall postoperative complication rate with VL compared to open. Length of stay was shorter for the VL cohort by a median of 1 day [mean, 8.1 vs. 9.7 days, respectively (P<0.0001)] and more VL patients were discharged directly to home (74.5% vs. 67.2%) rather than to a transitional healthcare facility (21.6% vs. 30.1%, respectively; P=0.0037). There was no difference in postoperative or 30-day mortality rates between open and VL patients.
Relevant complications are presented in Table 6; complication rates were comparable between the VL and open cohorts. There was a trend toward fewer postoperative transfusions in the VL cohort, but this did not reach statistical significance (P=0.08). Rates of acute respiratory failure were similar (10.9% VL vs. 12.4% OL, P=0.4571) as were rates of postoperative pneumonia (9.6% VL vs. 11.2% OL; P=0.4346).
Discussion
Recent data indicate the steady increase in proportion of octogenarians among lung cancer patients from 10% in 1996 to 17% in 2010 (13). In addition, the absolute number of octogenarians with lung cancer has been increasing, with a six-fold increase in patients with clinical stage I–II NSCLC from the late 1980s to the mid-2000s (14). However, increasing age is also associated with decreasing rates of surgery, despite numerous publications reporting acceptable morbidity and mortality rates in this higher risk patient population (2). The reluctance to offer surgery is due to the increased comorbidities in the elderly, with some series reporting 64–73% of their octogenarian surgical patients having at least one risk factor for surgery (15,16). Comorbidities in the elderly are associated with adverse outcomes, with one study reporting a 10% decline in postoperative survival in patients with 2 or more comorbidities (1). However, over the last two decades, the surgical care of octogenarians has improved with better outcomes, warranting an updated analysis (14).
This study is a current analysis of perioperative outcomes in octogenarians undergoing lobectomy and describes the largest matched series to date of minimally invasive lobectomy outcomes in this elderly group. Real-world data describing robotic-assisted, VATS, and open techniques show that open thoracotomy is still the most commonly performed approach in this elderly population (45%), followed by VATS (40%), and robotic-assisted lobectomy (15%). VATS and robotic-assisted approaches comprise 55% of all lobectomies in octogenarians, indicating that when the two techniques are combined, minimally invasive approaches have been adopted in the majority of cases for this higher risk population.
Overall, minimally invasive surgery in the octogenarian population was associated with improved outcomes compared to open thoracotomy. Despite the longer operating-room times with RL and VL, intraoperative complication rates were comparable to open cases. Albeit not a direct comparison, the conversion rate in the robotic-assisted cohort appears lower at 5% compared to 13.9% in the VL group. For both RL and VL patients, length of stay and the proportion of patients discharged directly to home compared favorably to the open cohort. These metrics indicate that both robotic-assisted and VATS techniques offer potential advantages to this higher risk population and are associated with faster recovery than open surgery.
Overall postoperative complications were significantly lower in the robotic-assisted group compared to the open group; however, a similar finding was not observed in the VATS versus open comparison. Of note, there was a significant difference in rates of post-operative bleeding and need for transfusion favoring RL versus OL, a disparity not seen in the VL versus OL comparison. The reason for differences in these outcomes could not be obtained from the dataset. One possibility, however, is that VATS lobectomy may represent a relatively heterogeneous set of approaches that, in many cases, may involve rib spreading or mini thoracotomy, and which may offset some advantages of a true VATS technique. The experience of all of the surgeons is also unclear from the dataset, and it is possible the robotic surgeons were more experienced. The published literature comparing VATS to open lobectomy in the octogenarian population has yielded mixed results. Some authors have reported significantly better outcomes with fewer complications when comparing VATS to open (7,8). McVay and colleagues reported the largest VATS lobectomy series (n=153) in the octogenarian population (17) with excellent outcomes (1% conversion rate and 18% postoperative complication rate). However, other investigators have compared VATS to open cases in octogenerians and reported that postoperative complications were similar between both groups (4,18).
The Premier database represents real-world outcomes from a diverse group of surgeons and hospitals. In the current study, nearly 40% of robotic-assisted cases and more than 50% of VATS cases came from community hospitals. Although we did not compare outcomes with younger patients, the outcomes in the octogenarian population in this analysis can be benchmarked with a recent publication on all elective lobectomies during the same study period. In that study, Oh et al. identified the overall postoperative complication rate ranged from 34% for robotic-assisted lobectomy to 46% for open lobectomy with a length of stay ranging from a median of 5 days for robotic lobectomy to 7 days with open thoracotomy (10). In comparison to the current data in octogenarians, it is apparent that the elderly population is associated with more postoperative complications and longer length of stay. In addition, in the overall lobectomy population from that previous publication, approximately 90% of patients were discharged directly home following lobectomy, compared to 67–78% in the octogenarian cohort reported here (10). These comparative data are valuable when discussing with octogenarian patients the perioperative risks and expectations following surgery. With overall postoperative complications ranging from 40% to 50% across all surgical techniques in the octogenarian population, there is certainly a need for improvement in the postoperative management of these patients, as well as judicious patient selection.
Overall, postoperative mortality outcomes from lobectomy in the octogenarian population are acceptable as reported in this database, ranging from 2.2–4.3%, and do not differ significantly between surgical approaches. It is worth noting that the mortality rate is lower than what has been previously published, which ranges from 6.3–9.4% (1,15,19,20). Although this improvement in mortality reported here likely reflects better patient selection over time, it may also represent a better general health state of modern octogenarian patients than in the past as well as improved surgical techniques. Nonetheless, the postoperative mortality after elective lobectomy in the general population was 1.2–2.2% during the same period, so there does appear to be an inherent increased risk in this older cohort (10). This study is limited to 30-day mortality, and it would be of interest to compare 90-day mortality in octogenerians who have lobectomy relative to the overall population since this metric may be more informative in the frail patient (21). In the era of increasing SBRT use for lung cancer in the elderly population, these real-world outcomes indicate that minimally invasive lobectomy in the octogenarian population can be accomplished safely with low perioperative mortality and should give pause to the assumption that very elderly patients are inoperable.
This study does have several inherent limitations, including its retrospective nature and the use of a large national insurance database. As an example, the Premier database does not include information on cancer stage or size of tumor, allowing the possibility that the open cohort comprised patients with larger tumors or more lymphadenopathy. Although propensity matching was used to compare distinct matched pairs of robotic-assisted and VATS patients versus OL, we could not simultaneously perform a third comparison between RL vs. VATS. This would have resulted in far fewer included patients, introduced significant statistical complexity, instability and variability, and ultimately would have compromised the validity of the current study methodology and findings. Thus, we elected to omit this comparison as the aim of the study was to compare minimally invasive techniques to open.
In conclusion, this current analysis of real-world outcomes for lobectomy in octogenarians shows that minimally invasive techniques now comprise more than half of all cases in this patient population. Both robotic-assisted and VATS lobectomy cohorts were associated with improved lengths of stay and discharges to home compared to open lobectomy, and robotic-assisted lobectomy was associated with fewer postoperative complications, including bleeding and need for blood transfusions, compared to open. Nationwide there are improving postoperative mortality rates in octogenarian patients compared to older published reports. These findings suggest that minimally invasive lobectomy is a viable option in octogenarians with improved outcomes compared to open thoracotomy. The robotic-assisted approach may yield the most favorable complication profiles, although more directed comparisons between robotic-assisted and VATS procedures are needed to better assess this.
Acknowledgments
The authors wish to thank Wainwright Medical Communications for editorial support. This work was supported by Intuitive Surgical, Inc.
Footnote
Conflicts of Interest: Dr. Sarkaria receives speaking and education honoraria from Intuitive Surgical; Dr. Oh was a past consultant for Ethicon and Covidien and is a part-time medical advisor for Intuitive Surgical; Dr. Gorrepati and Ms. Mehendale are employees of Intuitive Surgical.
Ethical Statement: No institutional review board approval was required for this study [45 CFR 46.101(b)(4)].
References
- Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg 2007;205:729-34. [Crossref] [PubMed]
- Blanchard EM, Arnaoutakis K, Hesketh PJ. Lung cancer in octogenarians. J Thorac Oncol 2010;5:909-16. [Crossref] [PubMed]
- Available online: www.ssa.gov/OACT/population/longevity.html, Accessed January 31, 2018.
- Feczko A, McKeown E, Wilson JL, et al. Assessing survival and grading the severity of complications in octogenarians undergoing pulmonary lobectomy. Can Respir J 2017;2017:6294895. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153-9. [Crossref] [PubMed]
- Chang JY, Suresh SS, Marinus AP, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. A model for morbidity after lung resection in octogenarians. Eur J Cardiothorac Surg 2011;39:989-94. [Crossref] [PubMed]
- Port JL, Mirza FM, Lee PC, et al. Lobectomy in octogenarians with non-small cell lung cancer: ramifications of increasing life expectancy and the benefits of minimally invasive surgery. Ann Thorac Surg 2011;92:1951-7. [Crossref] [PubMed]
- Igai H, Takahashi M, Ohata K, et al. Surgical treatment for non-small cell lung cancer in octogenarians—the usefulness of video-assisted thoracic surgery. Interact Cardiovasc Thorac Surg 2009;9:274-7. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent premier data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009;47:626-33. [Crossref] [PubMed]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-55. [Crossref]
- Kaniski F, Enewold L, Thomas A, et al. Temporal patterns of care and outcomes of non-small cell lung cancer patients in the United States diagnosed in 1996, 2005, and 2010. Lung Cancer 2017;103:66-74. [Crossref] [PubMed]
- Ganti AK, Shostrom V, Alorabi M, et al. Early stage non-small-cell lung cancer in octogenarian and older patients: a SEER database analysis. Clin Lung Cancer 2016;17:285-91. [Crossref] [PubMed]
- Dominguez-Ventura A, Cassivi SD, Allen MS, et al. Lung cancer in octogenarians: factors affecting long-term survival following resection. Eur J Cardiothorac Surg 2007;32:370-4. [Crossref] [PubMed]
- Hino H, Murakawa T, Ichinose J, et al. Results of lung cancer surgery for octogenarians. Ann Thorac Cardiovasc Surg 2015;21:209-16. [Crossref] [PubMed]
- McVay CL, Pickens A, Fuller C, et al. VATS anatomic pulmonary resection in octogenarians. Am Surg 2005;71:791-3. [PubMed]
- Koizumi K, Haraguchi S, Hirata T, et al. Lobectomy by video-assisted thoracic surgery for lung cancer patients aged 80 years or more. Ann Thorac Cardiovasc Surg 2003;9:14-21. [PubMed]
- Damhuis R, Coonar A, Plaisier P, et al. A case-mix model for monitoring of postoperative mortality after surgery for lung cancer. Lung Cancer 2006;51:123-9. [Crossref] [PubMed]
- Ginsberg RJ, Hill LD, Eagan RT, et al. Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 1983;86:654-8. [PubMed]
- Pezzi CM, Mallin K, Mendez AS, et al. Ninety-day mortality after resection for lung cancer is nearly double 30-day mortality. J Thorac Cardiovasc Surg 2014;148:2269-77. [Crossref] [PubMed]