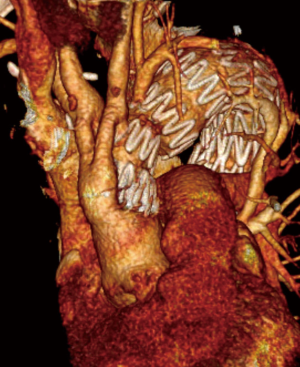
Off pump hybrid extra-anatomic techniques for aortic arch repair—own experience
Introduction
Twelve percent of thoracic and thoracoabdominal aneurysms (TAA) with diameter exceeding 6 cm rupture without intervention during 1-year follow-up and 50% of medically treated patients die within 5 years after such diagnosis (1,2). A huge progress in endovascular medicine have also had marked impact on the management of aortic arch pathologies in the last few years. In spite of this fact, outcomes of patients with extensive aortic diseases, including aortic arch and many co-morbidities are still associated with high mortality and morbidity (3).
Historically, open surgical techniques developed by Borst in 1983 underwent evolution from classic open surgery to open stent grafting technique (OSG) commonly known as frozen elephant trunk (FET) or stented elephant trunk (4). The aforementioned procedures are performed through sternotomy and cardiopulmonary bypass (CPB) with/without deep hypothermic circulatory arrest (DHCA) with some forms of cerebral perfusion have to be applied (5). Although the OSG techniques have been shown to decrease a rate of phrenic or recurrent laryngeal nerve injuries and reduced risk of other postoperative complications, they are still linked to early mortality 5% to 15% even in the high-volume cardiovascular centers (6-11).
The first procedures of extra-anatomic aortic arch repair that comprise of vascular transposition and endovascular stent graft implantation were performed by Volodos in 1991 but described in details by Bergeron in 2003 (11-13). They are considered as minimally invasive and done without CPB employment. Thus, they seem to be intriguing alternative to the OSG procedures, particularly in high-risk patients such as elderly, subjects with severe co-morbidities and those who are suboptimal candidates for open surgeries because of previous heart and vascular interventions (11-13).
Our team-initiated hybrid procedures many years ago in a result of excellent cooperation between cardiac, vascular surgeons and interventional radiologists. In this study, early and late outcomes of patients with aortic arch pathologies treated by means of hybrid methods were evaluated.
Methods
Patients
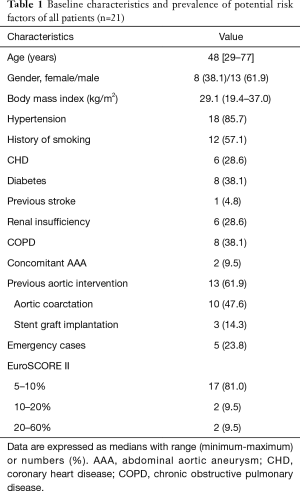
In the last 12 years, 135 patients have been treated in the Department of Cardiac Surgery and Transplantology due to thoracic aortic pathologies. In 21 cases (15.6% of all patients who underwent stent grafts implantation) hybrid procedures were performed. This group consisted of 8 women and 13 men with the median age of 48 (ranged 29 through 77 years). Their basic demographic parameters, concomitant disorders and other potential risk factors of early mortality according to EuroSCORE are listed in Table 1. In addition to high-risk patients, there were also individuals who did not agree for open surgical procedures in the other centers.
Full table
According to the rules of Local Bioethical Committee of our university the Statement of Ethics Approval is not required for retrospective data analysis of patients treated with the use of standard methods. Thus, the authors of this study did not apply for such approval.
Preoperative examination
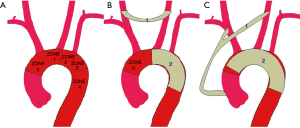
Before surgery, both final diagnosis of aortic pathology and qualification for surgery were established on the base of computed tomography (CT) scans with contrast medium [CT angiography (CTA)]. The diameter of aorta as well as the anatomical relations of aortic arch branches were assessed. In addition, the intended locations of both the proximal and distal ends of stent graft (LZ, Landing Zone) were determined on the base of disease-free aortic segments of at least 20 mm in length (Figure 1) (8,9). In every case, the diameter and quality of cervical and spinal arteries were assessed with Doppler ultrasound examination.
Surgical part of hybrid procedure
All operations were performed under general anaesthesia. Before surgery, the patients received 5,000 IU of heparin. In all individuals, antibiotic prophylaxis was used according to the approved hospital protocol (Cephazolin at a dose of 1.0 g every 8 hours for 2 days).
Hemi-arch transposition
Due to the inability to secure the stent graft deployment in LZ 1 (i.e., between brachiocephalic trunk and the left common carotid artery, see Figure 1), a decision to perform a two-step procedure was made. After a carotid-to-carotid anastomosis with Dacron vascular prosthesis (8 mm in diameter), a stent graft distally to brachiocephalic trunk was implanted. It covered the orifices of both the left common carotid and left subclavian arteries (Figure 2).
Total-arch transposition
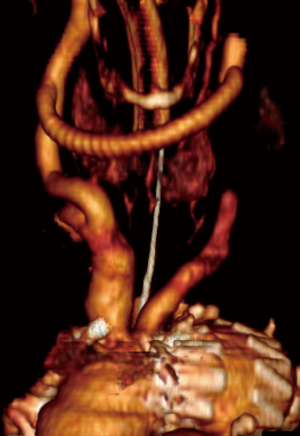
A two-step procedure was done from upper right ministernotomy (in the II intercostal space) extended into the left neck. First, bifurcated Dacron 16/8 mm (at the very beginning 14/7 mm) vascular prostheses were sewn with side clamp to the ascending aorta then end-to-side or end-to-end manner to brachiocephalic trunk and left common carotid artery. During the same procedures or after 14 days, the stent grafts were implanted into the ascending aorta with the proximal end then distally to previously created surgical anastomoses. They covered all the branches of the aortic arch and enlarged aneurysm sac (LZ 0) (Figures 3,4). In every case, near infrared spectroscopy (NIRS) was applied for monitoring of brain tissue oxygenation.
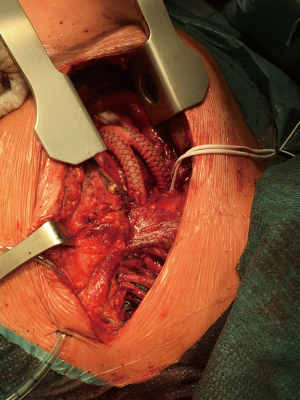
Stent graft implantation procedure
Nine hybrid procedures were performed in two steps, the first one in the operating room and the latter one in the vascular laboratory. Five of them were carried out with a 2-week break. Twelve single-step hybrid interventions were done exclusively in the hybrid operating room. Two experienced cardiac surgeons and one interventional radiologist performed all procedures.
Each endovascular procedure was done with the use of endovascular operating C-arm unit (Allura, Philips Medical Systems, Best, The Netherlands). Available image intensifier field sizes were 17, 23 and 31 cm. Generally, contrast medium was injected by automatic syringe. In all but two ionic contrast was used. Non-ionic one was reserved for patients with allergy and pre-existing renal failure. Five to 10 series were done, depending on clinical indications and procedure complexity. No particular spinal cord protection methods were employed.
Thoracic stent grafting was carried out from a femoral approach. The left common femoral artery was punctured by Seldinger’s method and the right one was exposed surgically. A 6-F straight catheter was introduced into the ascending aorta on a pigtail 5-F straight one with side holes. The prosthesis was introduced into the thoracic aorta over an Amplatz 0.35 guide wire. Oversized (usually up to 10%) grafts were implanted for treatment of aneurysm but in case of dissections oversizing was avoided.
Finally, to confirm appropriate stent graft position and to exclude any endoleaks, control digital subtraction angiography (DSA) was done.
Postoperative evaluation
The acetylsalicylic acid at a dose of 150 mg per day was administrated in every case as antiplatelet regimen. Each patient was followed up regularly in the Cardiac Surgical Outpatient Clinic. During the follow-up period that lasted from 6 to 118 months (median 56 months) and was completed by 100% of survivors, the imaging examinations (including CTA) were done 1, 6 and 12 months after procedures and then once a year.
Data management and analysis
The data analysis was performed anonymously. First, the quantitative variables were checked for normality by means of the W Shapiro Wilk test. Because they did not satisfy criteria of normal distribution, they are presented as the medians with range (minimum–maximum). Categorical data are expressed as number (n) with percent (%).
Results
Indications
In 14 (66.7%) patients hybrid procedures were applied to treat true aneurysms of aortic arch (TAA), in 5 (23.8%) Stanford type B dissections and in two cases aorto-oesophageal fistulas (9.5%). In 13 cases (61.9%) procedures on the aortic arch were reinterventions after previous invasive treatment (see Table 2).
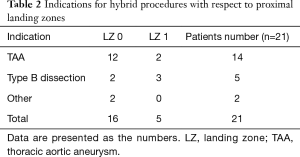
Full table
Surgical stage
The total time of surgical part was 120 minutes (90–200 minutes).
Stent grafts implantation
In all cases, Zenith stent grafts (Cook Medical, Bloomington, Inc., USA) were implanted. In 16 cases, the proximal end of the stent graft was positioned in LZ 0, in the others five in LZ 1.
The median length of grafts was 134 mm (115–202 mm) and diameter 26 mm (24–32 mm), respectively (see Table 3).
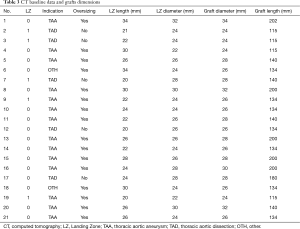
Full table
The median total time of endovascular procedures was 87 minutes (70–130 minutes), fluoroscopy time did not exceed 25 minutes and the maximum dose of 1 Gy was not reached.
In-hospital outcomes
All patients survived the procedure and nobody developed intraprocedural complications. The control angiography demonstrated laminar flow of blood in the aortic arch and its branches as well as complete exclusion of the aortic pathologies following deployment of stent grafts (100% of technical success) (Figures 2-4).
In-hospital mortality was 9.5%. Two patients died as a consequence of multi-organ failure (MOF), one on 19th and another on 24th postoperative day. The earlier one was operated on due to aorto-oesophageal fistulas. He survived procedure of total aortic arch debranching but developed sepsis that eventually led to fatal MOF and death. After the operation, one patient had symptoms of cerebral stroke whereas in another one spinal cord ischemia was diagnosed. The latter one presented chronic paresis with areflexia of lower extremities and neurogenic bladder. The total prevalence of early neurological adverse events was 9.5% (see Table 4).
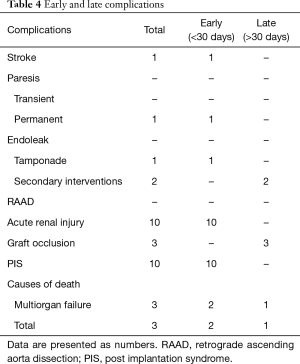
Full table
In one subject soon after stent graft implantation cardiac tamponade treated successfully with pericardial sack drainage was noted. The Velazquez syndrome, called also post-implantation syndrome (PIS) and characterized by fever and leukocytosis but without following markers of infection such as increased concentrations of high sensitivity C-reactive protein and procalcitonin, was observed in ten cases. No other complications associated with the implantation of the stent graft during perioperative period were noticed.
In our group, median length of stay in the intensive care unit (ICU) was 1 day whereas total hospitalization time 6 days.
Late follow-up
The follow-up period ranged from 6 to 118 months and was completed by all patients (100%). In the last follow-up imaging studies, the correct prosthesis position and function (i.e., complete exclusion of aortic wall pathologies without endoleak evidences) were observed in all individuals. There were neither graft migration nor its break cases. Moreover, no evidences of aorta dilatation and the formation of false aneurysms were noted throughout this period.
During the follow-up period one patient died 3 years after hybrid procedure as reintervention due to infected aorto-oesophageal fistula. This patient survived operation and was discharge 6 weeks later. At that time, he was treated with wide-spectrum intravenous antibiotics. Unfortunately, in spite of oral therapy he developed sepsis many months after surgery and eventually died due to MOF.
In our observation complications were similar in the total and hemiarch groups. Only in the total arch group the grafts arm occlusion was more frequent. Thrombosis was observed in three individuals (two cases in total-arch vs. one case in hemi-arch). In one case after hemi-arch debranching, vascular graft thrombosis due to its collapse was observed. In two others prostheses arms occlusions following total-arch debranching were confirmed in the imaging studies. It must be noted that all aforementioned vascular complications were diagnosed during the first years of our experience (Table 4).
Discussion
Significance of LZs
In 2002, introduction of “LZ” term and concept of five LZs enabled systematic planning and safe application of stent grafts in patients with complex aortic arch pathologies (14,15). During the 2004 Tokyo Consensus, conditions to provide stable and permanent placement of the proximal end of the prosthesis were formulated (16). The minimum length of fixation along the lesser curvature of the normal arch should be at least 20 mm. In our group only in two cases LZ length was equal to 20 mm whereas in the others was longer (see Table 3). It was postulated LZ aortic diameter larger than 38 or 40 mm markedly increased the risk of endoleak. The largest stent graft implanted by our team had diameter of 34 mm. Estimation of both radius and length of the aortic arch curvature have a significant impact on the stability of graft fixation, mainly in LZs 2 and 3. Maximum oversizing of the implanted prosthesis should not exceed 10% in TAA while should be avoided in type B dissection cases (17). In our practice we did follow aforementioned recommendations and it resulted in very promising early and late outcomes. Of note, nobody in our group had either endoleak or retrograde aortic dissection found within the follow-up period.
Placement of stent grafts in zones 1 and 0 requires additional vascular procedures, called either hemi- or total-arch debranching, respectively. Surgical details of these operations were described earlier. During the aforementioned operations, orifice of the left subclavian artery (LSA) is covered routinely. The meta-analysis of Hajibandeh et al. proved that the routine LSA revascularization neither decreased mortality nor reduced neurological adverse events among patients undergoing TEVAR with stent grafts implanted in LZ 2 (18). Only in 10% or less of such cases additional vascular interventions are necessary to restore proper blood supply to the central nervous system (CNS) (19). The absolute indications for them are as follow as hypoplastic or aberrant right subclavian artery (RSA), absent right vertebral artery, previously implanted left internal mammary artery to any of the coronary arteries, functioning left arm arteriovenous shunt for hemodialysis, aberrant origin and rare variants of the anatomical origin of the left vertebral artery (LVA) (18-24). Having in mind all such conditions, we analyzed very carefully imaging studies before all hybrid procedures. We are sure it enabled us to avoid any potential vascular complications related to obstruction of LSA orifice.
Treatment strategy
Currently, open surgery for aortic arch pathologies is still considered as a gold standard although early mortality is relatively high (3-11). There is no clear evidence as to the superiority of one technique over another. In our patients, we individually decided on a hybrid technique with debranching due to clinical indications, in patients who were re-operated, with comorbidities or older. There were also younger patients who did not accept the risk of extensive open surgery. There are no publications comparing open and hybrid techniques regarding only the pathology of the aortic arch. Although the results of open surgical treatment are still better, mortality may still reach 15–20%. In our group, hospital mortality is about 10%. The small size of our group is definitely the limitation.
Contrary to that De Rango et al. proved that hybrid techniques can be applied with high technical success rate (97.1%) but accompanied by acceptable mortality (5.8%) and low risk of neurological complications [stroke (3.8%) and spinal cord ischemia (2.9%)]. Moreover, detailed analysis of mid-term outcomes revealed a low prevalence of aortic disease- and intervention-related death and adverse events in the follow-up period (25).
Gaining experience led to opinion that hybrid aortic arch procedures should have been considered as less invasive alternatives, particularly applicable in elderly patients with severe co-morbidities, high-risk anatomy or previous cardiac surgery. Among them, subjects who require treatment in LZ 0 are the most challenging cases (26). In our group, the majority of patients were high-risk due to previous open surgical or endovascular procedures, their age, active infection or concomitant disorders (see Table 1).
We are aware that not all individuals are good candidates for hybrid aortic arch treatment. Even moderately dilated ascending aorta is considered as a risk factor of serious adverse events. The ascending aortic diameter larger than 37 mm was shown to increase risk of retrograde dissection (27). Analyzing De Rango report it was found that only 7% to 17% of patients treated through open surgical access would have met the anatomical criteria for hybrid intervention (25).
Brilliance and shadows
Open aortic arch repair procedures can be performed either by the two-stage elephant trunk approach (ET) or stented FET by a one-stage or open repair via clamshell incision (28). However, in high-risk patients morbidity and mortality can be significant, in spite of the advanced strategies of cerebral protection (29). Although classic approach employing CBP and DHCA was shown to reduce cerebral dysfunction, on the other hand they increased the risk of visceral ischaemia and postoperative bleeding due to hypothermia-induced coagulopathy (30). Invasive thoracosternotomy was associated with phrenic or recurrent laryngeal nerve injury and pulmonary complications (31). Eventually, all mentioned before adverse events led to prolonged in-hospital stay, including hospitalization at the ICU (26,32). Hybrid strategy enabled to avoid application of both CPB and DHCA as well as excessive surgical approach. The access for endovascular step can be femoral vessels or even in particular cases directly ascending aorta through minimally invasive upper ministernotomy (28).
We are aware the novel therapeutic options are not free from complications. Although endoleak prevalence may be up to 30% (33), in our opinion type 1 endoleak can be avoided by a proper selection of LZ and stent graft diameter whereas type 2 by native aortic branches ligation (28,32,34-36). Although high rates of endoleaks associated with hybrid debranching procedures have been reported the vast majority of them (i.e., up to 90%) were resolved within 6 months of follow-up (35).
The major consent of hybrid approach are neurologic complications, including severe cerebrovascular events and irreversible spinal cord injury (26,37). Careless manipulations of supra-aortic branches, introduction of numerous guide wires and catheters may lead to atheromatous or air embolism during procedures. Intraoperative monitoring with NIRS seems to be a crucial preventative measure. Late neurological adverse events may be linked to prosthetic branches occlusion (26-31). However, owing to our experiences we were able to reduce them to minimal level. In one of the first case, after hemi arch transposition we observed clotting and collapse of the Dacron prosthesis. Since then we started to use reinforced Gore-Tex prosthesis. After two incidences of bifurcated prosthetic arm occlusion at the very beginning we decided to change distal anastomosis method [end-to-end manner instead of end-to-side (38)] and to implant larger bifurcated prostheses (16/8 mm instead of 14/7 mm). The aforementioned measures helped us to resolve successfully problems of vascular graft occlusions.
Degeneration of the native ascending aorta may result in acute retrograde type A dissection. Although it is relative rare (prevalence 6%) but is associated with mortality rate as high as 42% (30). The guidelines of TEVAR suggest maximal oversizing up to 10% in TAA subjects and application of no oversized stent grafts in the cases of type B dissection. In group of patients no incidences of retrograde ascending aorta dissection were noted owing to strict compliance with mentioned above guidelines regarding oversizing (39).
The main advantages in high-risk population patients is the significant reduction in blood transfusion and of hospital stay. Our study supported earlier report with respect to ICU stay and hospitalization time (26).
We are aware it is not easy to compare the open and hybrid techniques for aortic arch repair, because of non-comparable patient groups. High-risk patients, elderly with significant co-morbidities with the history of cardiac or thoracic operations are usually qualified to hybrid procedures (28,31). The mortality rate ranges between 5% and 20% in open surgery and additionally 20% of the remainders have to be referred postoperatively to the departments for long-term nursing and intensive rehabilitation (35). Milewski et al. (40) reported no differences between open surgical and hybrid patients in terms of overall in-hospital mortality (16% vs. 11%, respectively), transient neurologic (11% vs. 11%) and permanent neurologic complications (9% vs. 13%, respectively). However, if the results were age-matched, the hybrid group provided superior outcomes. Mortality rate among elderly patients (with the age above 75 years) was 36% after standard open operation whereas 11% after hybrid procedures for aortic arch pathologies (36). One meta-analysis that confined 956 aortic arch debranching and 1,316 open surgical patients revealed favorable outcomes of hybrid procedure. The 30-day mortality was 11.9% vs. 9.5%, cerebrovascular events 7.6% vs. 6.2% and spinal cord injury 5.7% vs. 3.8%, respectively (26). It was supported by another meta-analysis that involved individuals treated between 2012 and 2018 (41).
There are not many studies that assessed the long-term fate of the aortic arch aneurysms after endovascular or hybrid procedures. De Rango et al. presented 1-, 3- and 5-year survival rates after hybrid arch treatment (89.0%, 82.8%, 70.9%, respectively) and the absence of aneurysm growth (defined as an increase in the aortic diameter over 5 mm) was observed in 96.7% of the cases during a 5-year follow up (42). Andersen et al. found that only 13% of hybrid patients during the first 3 years after procedures required re-interventions (43). The reported 5-year survival rate in Czerny et al. material was 96% (44).
Conclusions
Hybrid procedures on the aortic arch that comprise surgical and endovascular interventions have become attractive and safe therapeutic options with acceptable mortality and morbidity rate. They may be considered as the methods of choice in treatment of the elderly and high-risk patients since they do not require application of cardio-pulmonary bypass and excessive surgical access. However, favorable outcomes may be achieved only by experienced multidisciplinary team.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: According to the rules of Local Bioethical Committee of our university the Statement of Ethics Approval is not required for retrospective data analysis of patients treated with the use of standard methods. Thus, the authors of this study did not apply for such approval. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27. discussion 27-28.
- Younes HK, Davies MG, Bismuth J, et al. Hybrid thoracic endovascular aortic repair: pushing the envelope. J Vasc Surg 2010;51:259-66. [Crossref] [PubMed]
- Tian DH, Wan B, Di Eusanio M, et al. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg 2013;2:581-91. [PubMed]
- Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using ‘‘elephant trunk” prosthesis. Thorac Cardiovasc Surg 1983;31:37-40. [Crossref] [PubMed]
- Leontyev S, Misfeld M, Daviewala P, et al. Early- and medium-term results after aortic arch replacement with frozen elephant trunk techniques-a single center study. Ann Cardiothorac Surg 2013;2:606-11. [PubMed]
- Settepani F, Cappai A, Basciu A, et al. Outcome of open total arch replacement in modern era. J Vasc Surg 2016;63:537-45. [Crossref] [PubMed]
- Sachs T, Pomposelli F, Hagberg R, et al. Open and endovascular repair of type B aortic dissection in the Nationwide Inpatient Sample. J Vasc Surg 2010;52:860-6. [Crossref] [PubMed]
- Cowan JA, Mimick JB, Henke PK, et al. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg 2003;37:1169-74. [Crossref] [PubMed]
- Patel VI, Mukhopadhyay S, Ergul E, et al. Impact of hospital volume and type on outcomes of open and endovascular repair of descending thoracic aneurysms in the United States Medicare population. J Vasc Surg 2013;58:346-54. [Crossref] [PubMed]
- Chikwe J, Cavallaro P, Itahgaki S, et al. National outcomes in acute aortic dissection: in uence of surgeon and institutional volume on operative mortality. Ann Thorac Surg 2013;95:1563-9. [Crossref] [PubMed]
- Papakonstantinou NA, Baikoussis NG, Dedeilias P, et al. Cardiac surgery or interventional cardiology? Why not both? Let’s go hybrid. J Cardiol 2017;69:46-56. [Crossref] [PubMed]
- Volodos' NL, Shekhanin VE, Karpovich IP, et al. A self-fixing synthetic blood vessel endoprosthesis. Vestn Khir Im I I Grek 1986;137:123-5. [PubMed]
- Bergeron P, De Chaumaray T, Gay J, et al. Endovascular treatment of thoracic aortic aneurysms. J Cardiovasc Surg (Torino) 2003;44:349-61. [PubMed]
- Criado FJ, Clark NS, Barnatan MF. Stent graft repair in the aortic arch and descending thoracic aorta: a 4-year experience. J Vasc Surg 2002;36:1121-8. [Crossref] [PubMed]
- Criado FJ, Abul-Khoudoud OR, Domer GS, et al. Endovascular repair of the thoracic aorta: lessons learned. Ann Thorac Surg 2005;80:857-63. [Crossref] [PubMed]
- Mitchell RS, Ishimaru S, Criado FJ, et al. Third International Summit on Thoracic Aortic Endografting: lessons from long-term results of thoracic stent-graft repairs. J Endovasc Ther 2005;12:89-97. [Crossref] [PubMed]
- Puślecki M, Buczkowski P, Perek B, et al. Hybrid procedures for aortic arch repair. Kardiochir Torakochirurgia Pol 2011;4:438-44.
- Hajibandeh S, Hajibandeh S, Antoniou SA, et al. Meta-analysis of left subclavian artery cowerage with and without revascularization in thoracic endovascular repair. J Endovasc Ther 2016;23:634-41. [Crossref] [PubMed]
- Belczak SQ, Silva ES, Klajner R, et al. Type II endoleaks, left-arm complications, and need of revascularization after left subclavian artey coverage for thoracic aortic aneurysm endovascular repair: a systematic review. Ann Vasc Surg 2017;41:294-9. [Crossref] [PubMed]
- Gravereaux EC, Faries PL, Burks JA, et al. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. J Vasc Surg 2001;34:997-1003. [Crossref] [PubMed]
- Moore RD, Brandschwei F. Subclavian-to-carotid transposition and supracarotid endovascular stent graft placement for traumatic aortic disruption. Ann Vasc Surg 2001;15:563-6. [Crossref] [PubMed]
- Peterson BG, Eskandari MK, Gleason TG, et al. Utility of left subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg 2006;43:433-9. [Crossref] [PubMed]
- Riesenman PJ, Farber MA, Mendes RR, et al. Coverage of the left subclavian artery during thoracic endovascular aortic repair. J Vasc Surg 2007;45:90-4. [Crossref] [PubMed]
- Chiesa R, Melissano G, Marrocco-Trischitta MM, et al. Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J Vasc Surg 2005;42:11-7. [Crossref] [PubMed]
- De Rango P, Ferrer C, Coscarella C, et al. Contemporary comparison of aortic arch repair by endovascular and open surgical reconstructions. J Vasc Surg 2015;61:339-46. [Crossref] [PubMed]
- Moulakakis KG, Mylonas SN, Markatis F, et al. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg 2013;2:247-60. [PubMed]
- Vallabhajosyula P, Szeto WY, Desai N, et al. Type II arch hybrid debranching procedure. Ann Cardiothorac Surg 2013;2:378-86. [PubMed]
- Kollias VD, Lozos V, Angouras D, et al. Single-stage, off-pump hybrid repair of extensive aneurysms of the aortic arch and the descending thoracic aorta. Hellenic J Cardiol 2014;55:355-60. [PubMed]
- Patel HJ, Nguyen C, Diener AC, et al. Open arch reconstruction in the endovascular era: analysis of 721 patients over 17 years. J Thorac Cardiovasc Surg 2011;141:1417-23. [Crossref] [PubMed]
- Kent WD, Appoo JJ, Bavaria JE, et al. Results of type II hybrid arch repair with zone 0 stent graft deployment for complex aortic arch pathology. J Thorac Cardiovasc Surg 2014;148:2951-5. [Crossref] [PubMed]
- Faulds J, Sandhu HK, Estrera AL, et al. Minimally Invasive Techniques for Total Aortic Arch Reconstruction. Methodist Debakey Cardiovasc J 2016;12:41-4. [Crossref] [PubMed]
- Canaud L, Gandet T, Ozdemir BA, et al. Hybrid Aortic Repair of Dissecting Aortic Arch Aneurysm after Surgical Treatment of Acute Type A Dissection. Ann Vasc Surg 2016;30:175-80. [Crossref] [PubMed]
- Antoniou GA, El SK, Hamady M, et al. Hybrid treatment of complex aortic arch disease with supra-aortic debranching and endovascular stent graft repair. Eur J Vasc Endovasc Surg 2010;39:683-90. [Crossref] [PubMed]
- Cochennec F, Tresson P, Cross J, et al. Hybrid repair of aortic arch dissections. J Vasc Surg 2013;57:1560-7. [Crossref] [PubMed]
- Leacche M, Umakanthan R, Zhao DX, et al. Surgical update: hybrid procedures, do they have a role? Circ Cardiovasc Interv 2010;3:511-8. [Crossref] [PubMed]
- Shirakawa Y, Kuratani T, Shimamura K, et al. The efficacy and short-term results of hybrid thoracic endovascular repair into the ascending aorta for aortic arch pathologies. Eur J Cardiothorac Surg 2014;45:298-304; discussion 304. [Crossref] [PubMed]
- Bavaria J, Vallabhajosyula P, Moeller P, et al. Hybrid approaches in the treatment of aortic arch aneurysms: postoperative and midterm outcomes. J Thorac Cardiovasc Surg 2013;145:S85-90. [Crossref] [PubMed]
- Czerny M, Schmidi J, Carrel T, et al. Hybrid aortic arch repair. Ann Cardiothorac Surg 2013;2:372-7. [PubMed]
- Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41. [Crossref] [PubMed]
- Milewski RK, Szeto WY, Pochettino A, et al. Have hybrid procedures replaced open aortic arch reconstrution in high-risk patients? A comparative study of elective open arch debranching with endovascular stent graft placement and conventional elective open total and distal aortic arch reconstruction. J Thorac Cardiovasc Surg 2010;140:590-7. [Crossref] [PubMed]
- Papakonstantinou NA, Antonopoulos CN, Baikoussis NG, et al. Aortic Arch Reconstruction: Are Hybrid Debranching Procedures a Good Choice? Heart Lung Circ 2018;27:1335-49. [Crossref] [PubMed]
- De Rango P, Cao P, Ferrer C, et al. Aortic arch debranching and thoracic endovascular repair. J Vasc Surg 2014;59:107-14. [Crossref] [PubMed]
- Andersen ND, Williams JB, Hanna JM, et al. Results with an algorithmic approach to hybrid repair of the aortic arch. J Vasc Surg 2013;57:655-67. [Crossref] [PubMed]
- Czerny M, Weigang E, Sodeck G, et al. Targeting landing zone 0 by total arch rerouting and TEVAR: midterm results of a transcontinental registry. Ann Thorac Surg 2012;94:84-9. [Crossref] [PubMed]