
Percutaneous closure of patent foramen ovale under transthoracic echocardiography guidance—midterm results
Introduction
Patent foramen ovale (PFO), a fetal anatomy remnant, is one of the most common congenital cardiac malformations, with a prevalence of up to 20%. Due to paradoxical embolism (PE), i.e., passage of venous clots, PFO is associated with cryptogenic stroke (CS), transient ischemic attack (TIA) and other embolic complications (1). Treatment of PFO includes medical (oral antiplatelet agents, coumadin, or a combination thereof) and interventional (open-heart surgery and percutaneous closure) approaches. However, open-heart surgery is no longer recommended because of its more traumatic nature, perioperative complications and requirement for cardiopulmonary bypass. Medical therapy has limited effectiveness in preventing recurrent cerebrovascular events and it carries a substantial long-term risk of bleeding and gastrointestinal complications (2).
Whether occluder implantation of PFO reduces the risk of recurrence of ischemic stroke in patients who have had a CS remains controversial. Previous multicenter clinical trials demonstrated that closure of PFO combined with antiplatelet therapy was associated with a lower rate of recurrent ischemic stroke than medical therapy alone during follow-up (3-5). More recently in a meta-analysis, Ntaios et al. indicated that closure of PFO in patients with CS or TIA results in less frequent ischemic stroke recurrence compared with medical treatment alone (6). In a review, Alkhouli et al. concluded that the role of PFO closure in stroke prevention is now firmly established (7). Therefore, percutaneous closure, with less trauma and quicker recovery, is currently considered first line treatment for symptomatic PFOs. However, it is routinely performed under fluoroscopy guidance with exposure to radiation, which is potentially harmful to doctors and patients (8,9), and to contrast media with risk of renal failure in some patients (10). The present study thus aimed to assess the safety and efficacy of percutaneous PFO closure under guidance of transthoracic echocardiography (TTE) as the only imaging modality.
Methods
Subjects
The present study retrospectively enrolled a total of 52 consecutive patients who underwent TTE-guided percutaneous closure of PFO at our hospital from June 2015 to September 2017. Patients were eligible for inclusion if they met all of the following criteria: (I) presence of PFO defined as right-to-left shunt through a gap between the septum primum and secundum documented by contrast transesophageal echocardiography (TEE) using aerated colloid solution injected into an antecubital vein at the end of a standard Valsalva maneuver, and without other cardiac anomalies; (II) presence of symptoms such as headache, dizziness and a diagnosis of CS or TIA by neurologist; and (III) PFO related cerebral infarction, ischemic stroke or recurrent TIA despite medical therapy. Patients were excluded if they met any of the following criteria: (I) presence of identifiable cause of ischemic event such as carotid or intracranial artery stenosis, cerebrovascular thrombosis, atherosclerotic plaque at the aortic arch or a history of atrial fibrillation (AF); and (II) presence of other heart disease or cardiac malformations requiring open-heart surgery. The study protocol was approved by the Institutional Ethics Committee of Fuwai Hospital and all patients provided written informed consent prior to recruitment.
Definitions
PFO size was assessed in all patients by contrast TEE with sustained Valsalva maneuver. Shunt was defined as the appearance of agitated saline contrast (bubbles) in the left atrium (LA) within 3 cardiac cycles of right atrial opacification. Shunt was graded as mild, moderate or severe if 3–9, 10–30 or >30 contrast bubbles appeared in the LA, respectively (11). Atrial septal aneurysm (ASA) was diagnosed as abnormally redundant interatrial septum (IAS) with an excursion of ≥10 mm during the cardiac cycle from the midline (12). The risk of paradoxical embolism (RoPE) scoring system was used to identify when stroke had a greater probability of being associated with PFO than being incidental (13).
Procedures and periprocedural treatment
The distance from the right parasternal third intercostal space to the puncture site was measured and defined as the “working length” (Figure 1).

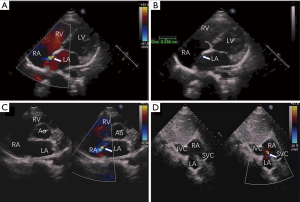
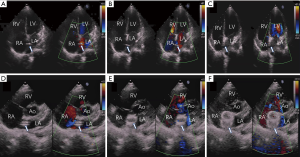
Firstly, the defect was detected by color doppler view of TTE, and the PFO was measured in the apical four-chamber view (Figure 2A,B,C,D). A 0.035-inch super-stiff guide-wire (Cook Lunderquist amplatz type extra stiff 260 cm) was inserted via the inferior vena cava to the right atrium (RA) under TTE guidance. A 6-Fr Cordis MPA-2 catheter was then advanced into the RA along the guidewire and the tip of the catheter was tracked by TTE. After the catheter passed through the PFO towards LA (Figure 3A), the guidewire, which was guided by the catheter, was then advanced through the PFO to LA (Figure 3B). The catheter was extracted, and a delivery sheath was selected according to the occluder’s diameter and was inserted through the PFO towards LA along the guidewire (Figure 3C,D). Under TTE monitoring, the left atrial side disk of the occluder (Starway Medical Technology Inc., Beijing, China 18/18 mm, 18/25 mm, 30/30 mm) was released (Figure 3E). With the occluder disk covering the left atrial side of the PFO, the delivery cable was fixed and the delivery sheath was retrieved. Finally, the right atrial side disk was released (Figure 3F). Immediately after occluder deployment, the apical four-chamber, parasternal short axis, and subcostal view (Figure 4A,B,C) were used to evaluate the device position and potential impingement of the device on adjacent cardiac structures. Color Doppler assessment also was performed in these views to detect residual shunts, coronary sinus return, systemic and pulmonary venous return, as well as to assess the competence of atrioventricular valves (Figure 4). When the occlude was confirmed to be implanted in the correct position with a good shape, it was released by rotating the cable counterclockwise under TTE guidance.
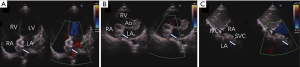
After release, reassessment was performed in these views. Finally, the delivery sheath was removed, and bandages were applied.
Follow-up and study endpoint
TTE, chest X-ray and electrocardiography (ECG) were used for assessment before discharge. The patients were followed up by clinical examination, TTE and ECG at 1, 3, 6 and 12 months after discharge and annually thereafter. The primary end point of the present study was a composite of recurrent stroke, TIA or peripheral embolism.
Statistical analysis
SPSS 20.0 for Windows (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. Continuous variables are expressed as mean ± standard deviation or median (interquartile range, 25% to 75%), and categorical variables as absolute value and percentage.
Results
Among the 52 patients studied, 55.8% were male, mean age was 34.0±13.0 (range, 10–59) years, mean body weight was 58.7±10.8 (range, 29.0–80.0) kg. A proportion of 7.7% had an ASA, 9.6% had a hypermobile IAS and 76.9% had a history of stroke, 23.1% of TIA, 36.5% of smoking, 21.2% of hypertension, 5.8% of diabetes mellitus, 17.3% of hypercholesterolemia, and none of coronary or peripheral artery disease. The baseline characteristics of all patients are shown in Table 1.
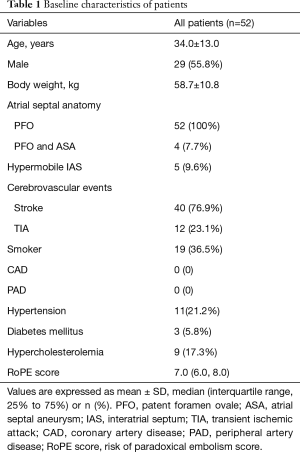
Full table
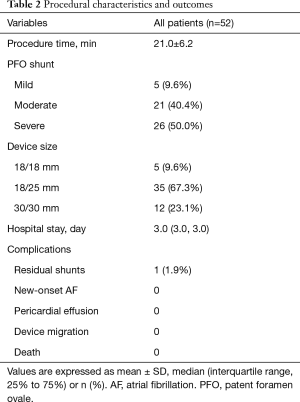
Of the 52 patients, 47 (90.4%) successfully underwent percutaneous PFO closure under TTE guidance. PFO shunt was graded as mild in 5 cases (9.6%), moderate in 21 cases (40.4%) and severe in 26 cases (50.0%). Median RoPE score was 7.0 (6.0, 8.0). Occluder size was 18/18 mm in 5 cases, 18/25 mm in 35 cases, and 30/30 mm in 12 cases. The mean procedure duration (from puncture to sheath removal) was 21.0±6.2 (range, 11–33) minutes. TEE-guidance was added for catheter advancement through PFO intraoperatively in 5 patients with thick chest walls due to limited ultrasound penetrability. A trivial residual shunt (less than 2 mm) was observed in only 1 patient (1.9%) immediately after the procedure, and it disappeared without additional management before discharge. No residual shunts of greater size occurred. None of the patients suffered serious complications such as pericardial effusion, peripheral vascular injury, cardiac perforation, valve regurgitation, occluder displacement, new-onset AF, malignant arrhythmia or death. Most patients were discharged within 3 days after the procedure. The median hospital stay was 3.0 days. The procedural characteristic and periprocedural outcomes are summarized in Table 2.
Full table
Follow-up information was available for all patients. At median 15.5 (11.3, 18.0) months follow-up, there were no deaths, recurrent stroke, TIA, peripheral embolism or other severe complications such as delayed pericardial effusion, heart blocks requiring pacemaker implantation, hospitalization related to PFO, or device related problems. By TTE, there were no new residual shunts, and the occluder was in good shape and position.
Discussion
In the present study on mostly lean patients, percutaneous PFO closure under TTE guidance as the only imaging tool yielded excellent clinical outcomes at midterm follow-up without stroke, TIA, death or peripheral embolism.
The results of the present study are consistent with those of previous ones in which percutaneous PFO closure significantly reduced the risk of recurrent stroke and TIA relative to medical therapy (14-17). Although fluoroscopic and angiographic guidance offers excellent visualization, exposure to radiation and contrast agents might pose greater risk to medical staff and patients (8,9), and radiation in children might significantly increase lifetime cancer risk, because of their immature organs and longer lifespan (18).
Compared with surgical closure, percutaneous PFO closure with only TTE as the imaging tool causes less trauma, avoids general anesthesia, endotracheal intubation and cardiopulmonary bypass with less perioperative complications. Compared with conventional fluoroscopy-guided percutaneous technique, TTE-guided closure requires no radiation exposure, obviating the need for large radiological imaging equipment, thereby reducing the economic burden on patients. Moreover, TTE as the only imaging modality in our study allowed to assess the impact of the closure device on cardiac valves, coronary sinus, pulmonary veins, and real-time monitoring of the occlude release process leading to device replacement if found to be incorrectly positioned (19), as documented in previous studies by our group on various cardiac anomalies (20,21).
Although the final success rate of PFO closure under TTE guidance as the only imaging tool was 90.4% (47/52) and the midterm outcome was satisfactory, this procedure is technically difficult and requires a relatively lengthy learning curve because of the fundamental differences in imaging displays. Traditionally, it is easy for interventionalists to determine the location of the catheter through fluoroscopy as the imaging tool. However, ultrasound detection involves various facets and might not accurately show the location of the catheter, rendering it more difficult to track the tip of the catheter or guidewire with TTE.
Based on the experience at our institution, the following items contribute to improving the safety of the TTE guided approach and to expediting the learning curve (20). First, an experienced team is required. The operator should have extensive knowledge of cardiac anatomy, with extensive experience in percutaneous treatment, and be able to perform the conventional fluoroscopy-guided PFO closure. Team members should be able to carry out open-heart surgery in case of emergency, and the sonographer should have clinical experience in TTE and be able to communicate seamlessly with the operator. Second, the working length should be measured and marked on the catheter; when the catheter with corresponding length is inserted, detection by TTE of catheter position in the RA can be facilitated by rotating the catheter. Third, accurate positioning is extremely important. The insertion distance of the delivery sheath should be 2–3 cm longer than that of the catheter and it should be marked after the catheter is removed. Moreover, use of appropriate devices is also important to ease procedure performance. With our increasing experience, the team also has gained important insights that have already helped to improve TTE guidance for PFO closure and to prepare this technique for worldwide clinical dissemination (21).
Study limitations
Firstly, the study has the limitations inherent to its single center design with a small sample size. Secondly, TTE guidance only might not be safe enough in complex cases such as multiple fenestration, stiff tunnel anatomy, redundant Eustachian valve and highly compliant PFO, which were not included in the present study. Thirdly, the rate of patients with ASA was extremely low (7.7%), which makes implantation of the occluder easier. A higher number of subjects with complex anatomies might pose a greater challenge for this technique. In addition, follow-up time was not sufficiently long to rule out late cerebrovascular events or other late complications.
Although several studies used TTE as imaging modality for validation during follow-up (21-24), and we added follow-up clinical evaluation including detailed neurological examination to detect possible recurrent TIA, stroke or thromboembolic events, validation of successful implantation using an established imaging modality in large prospective, multicenter randomized controlled trials with longer follow-up period is warranted.
Conclusions
Our study of mostly lean patients demonstrated that percutaneous PFO closure with TTE guidance as the only imaging tool yielded satisfactory midterm results. Larger prospective, randomized controlled multicenter trials including cases with more complex PFO presentations and greater weight are warranted to assess the long-term safety and efficacy of this fluoroscopy-free approach.
Acknowledgments
Funding: This work was supported by 13th-5-year National Key Research and Development Program of China (No. 2016YFC1302004) and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2017-I2M-4-001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Ethics Committee of Fuwai Hospital (No. 2016-579) and all patients provided written informed consent prior to recruitment.
References
- Yaghi S, Bernstein RA, Passman R, et al. Cryptogenic stroke. Circ Res 2017;120:527-40. [Crossref] [PubMed]
- Mas JL, Arquizan C, Lamy C, et al. For the patent foramen ovale and atrial septal aneurysm study group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001;345:1740-6. [Crossref] [PubMed]
- Mas JL, Derumeaux G, Guillon B, et al. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med 2017;377:1011-21. [Crossref] [PubMed]
- Saver JL, Carroll JD, Thaler DE, et al. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med 2017;377:1022-32. [Crossref] [PubMed]
- Søndergaard L, Kasner SE, Rhodes JF, et al. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med 2017;377:1033-42. [Crossref] [PubMed]
- Ntaios G, Papavasileiou V, Sagris D, et al. Closure of Patent Foramen Ovale Versus Medical Therapy in Patients With Cryptogenic Stroke or Transient Ischemic Attack: Updated Systematic Review and Meta-Analysis. Stroke 2018;49:412-8. [Crossref] [PubMed]
- Alkhouli M, Sievert H, Holmes DR. Patent foramen ovale closure for secondary stroke prevention. Eur Heart J 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Roguin A, Goldstein J, Bar O, et al. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol 2013;111:1368-72. [Crossref] [PubMed]
- Meinel FG, Nance JW, Harris BS, et al. Radiation risks from cardiovascular imaging tests. Circulation 2014;130:442-5. [Crossref] [PubMed]
- Maioli M, Toso A, Leoncini M, et al. Persistent renal damage after contrast-induced acute kidney injury: Incidence, evolution, risk factors, and prognosis. Circulation 2012;125:3099-107. [Crossref] [PubMed]
- Mas JL, Zuber M. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French Study Group on Patent Foramen Ovale and Atrial Septal Aneurysm. Am Heart J 1995;130:1083-8. [Crossref] [PubMed]
- Pearson AC, Nagelhout D, Castello R, et al. Atrial septal aneurysm and stroke: A transesophageal echocardiographic study. J Am Coll Cardiol 1991;18:1223-9. [Crossref] [PubMed]
- Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013;81:619-25. [Crossref] [PubMed]
- Hara H, Virmani R, Ladich E, et al. Patent foramen ovale: Current pathology, pathophysiology, and clinical status. J Am Coll Cardiol 2005;46:1768-76. [Crossref] [PubMed]
- Wahl A, Juni P, Mono ML, et al. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation 2012;125:803-12. [Crossref] [PubMed]
- Kim M, Kim S, Moon J, et al. Effect of patent foramen ovale closure for prevention on recurrent stroke or transient ischemic attack in selected patients with cryptogenic stroke. J Interv Cardiol 2018;31:368-74. [Crossref] [PubMed]
- Khan AR, Bin Abdulhak AA, Sheikh MA, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke. JACC Cardiovasc Interv 2013;6:1316-23. [Crossref] [PubMed]
- Johnson JN, Hornik CP, Li JS, et al. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation 2014;130:161-7. [Crossref] [PubMed]
- Kardon RE, Sokoloski MC, Levi DS, et al. Transthoracic echocardiographic guidance of transcatheter atrial septal defect closure. Am J Cardiol. 2004;94:256-60. [Crossref] [PubMed]
- Pan XB, Ouyang WB, Wang SZ, et al. Transthoracic echocardiography-guided percutaneous patent ductus arteriosus occlusion: A new strategy for interventional treatment. Echocardiography 2016;33:1040-5. [Crossref] [PubMed]
- Pan XB, Ou-yang WB, Pang KJ, et al. Percutaneous closure of atrial septal defects under transthoracic echocardiography guidance without fluoroscopy or intubation in children. J Interv Cardiol 2015;28:390-5. [Crossref] [PubMed]
- Takafuji H, Hosokawa S, Ogura R, et al. Percutaneous transcatheter closure of high-risk patent foramen ovale in the elderly. Heart Vessels 2019. [Epub ahead of print].
- Hildick-Smith D, Williams T, MacCarthy P, et al. Occlutech percutaneous patent foramen ovale closure: Safety and efficacy registry (OPPOSE). Int J Cardiol 2017;245:99-104. [Crossref] [PubMed]
- Oto A, Aytemir K, Ozkutlu S, et al. Transthoracic echocardiography guidance during percutaneous closure of patent foramen ovale. Echocardiography 2011;28:1074-80. [Crossref] [PubMed]


