Fractional exhaled nitric oxide was not associated with the future risk of exacerbations in Chinese asthmatics: a non-interventional 1-year real-world study
Introduction
Asthma is a chronic inflammatory disease of the airways characterized by variable airway obstruction and hyper-responsiveness. Although the majority of asthmatics fulfil symptomatic control, others, for a variety of reasons, still suffer from recurrent exacerbation (1). Asthma exacerbations generate considerable morbidity, having a great impact on the quality of life of patients, and are the main cause of healthcare utilization in asthmatics (2). Therefore, prevention of exacerbations is a major goal in the management of asthmatics.
It is accepted that poor asthma control is a factor predicting future exacerbations (3). Poor adherence to the use of inhaled corticosteroids (ICS) (4), previous exacerbations (5,6), low FEV1% predicted (7,8), and hospitalization or emergency-room visit (9) are also considered as major predictors of future asthma exacerbations.
Asthma control is generally reflected by reported symptoms and lung function indices. Measurement of fractional exhaled nitric oxide (FENO) is a quantitative, non-invasive, easy to perform, and safe method of measuring airway inflammation (10-12). The over-expression of inducible nitric oxide synthase is the major incentive for the increase of FENO of asthmatics (13,14). In general, the measurement of FENO aid in identifying type 2 airway inflammation with asthmatics (15,16). FENO provides additional benefits for asthmatics management, including but not limited to discover eosinophilic airway inflammation (12,17). Studies have shown that FENO is helpful to diagnose asthma (18). Furthermore, it has been shown that FENO level is associated with increased risk of exacerbation (15,19). Bjerregaard et al. demonstrated that high FENO at baseline was associated with an increased risk of future virus-induced exacerbations in patients with stable disease (15). Gelb et al. showed that combined FENO and FEV1% predicted can stratify the risk for exacerbations in patients being treated for asthma (20). Compared to the studies mentioned above in which the phenotype and severity of a disease were narrowly defined, non-interventional real-world studies which include patients across the full clinical spectrum of asthma may well be able to more fully represent the disease. As we know, no real-world study has assessed the association between FENO, spirometry indices, and the future risk of exacerbations specifically in Chinese asthmatics.
Thus, the purpose of the prospective study with a follow-up of 1 year was to assess factors associated with future exacerbations of Chinese asthmatics. In particular, we aimed to evaluate the roles of spirometry and FENO in predicting exacerbations.
Methods
Study design
Patients with chronic persistent asthma to whom at least used one ICS was dispensed as monotherapy (ICS alone) or in a fixed combination with a long-acting beta-agonist (LABA, ICS/LABA) were recruited from October 2016 to July 2017 from the respiratory clinic of Southern Medical University, Nanfang Hospital (Guangzhou, China). This study was approved by the ethics committee of the hospital (code number NFEC2016-052), and all subjects have given their written informed consent prior to participation in the study. In case of minors, parents were informed and gave written consent.
The clinical information including demographic data, smoking history, comorbidities, preceding medicine usage, asthma severity, and medicine adherence were collected. FENO measurements and spirometry indices were performed at baseline. After the initial visit, patients were seen four more times at 3-month interval. Patients were regularly contacted by phone calls or text messages to remind them that they are participating in the study, and of their clinic visits. All patients were followed by the same physician to decide whether they had exacerbations, during the study period. Exacerbations were evaluated using information modified from the Global Initiative for Asthma (GINA) guideline (21) and the American Thoracic Society/European Respiratory Society (ATS/ERS) (12). Exacerbations were defined as an episode of aggravating symptom needed parenteral corticosteroid, an increase in systemic corticosteroids from a stable maintenance dose, ≥3 days of oral corticosteroid (OCS) use, or hospitalization or emergency-room visit. Adherence was defined as the extent to which patients act in line with the prescribed interval and dosing regimen, according to the International Society for Pharmacoeconomics and Outcomes Research (22). Overall mean adherence was defined as the percentage of prescribed inhalations that were taken. This measure was divided into two categories (>80% and <80%); good adherence was defined as above 80% adherent (23,24). Asthma treatments were regulated according to the Chinese Asthma Prevention and Treatment guidelines 2016 (25). Briefly, if good asthma control has been achieved and maintained for at least three months, step-down of the treatment was taken into account. If asthmatics were in persistent symptoms and/or exacerbations, common problems such as inhaler technique, persistent allergen exposure, and compliance have been addressed, step-up of the treatment were considered.
Subjects
Two hundred and forty-six patients, at least 14 years old, and diagnosed according to GINA 2016 (21) and the Chinese Asthma Prevention and Treatment guidelines 2016 (25) were sequentially enrolled in the study. Inclusion criteria were patients diagnosed with asthma for at least 3 months and lived in Guangzhou for more than 2 years. Patients with chronic persistent asthma were staged according to their spirometry findings and the frequency of daytime or nighttime asthma symptoms according to the Chinese Asthma Prevention and Treatment guidelines 2016 (25). Clinical characteristics of the stages were as follows: stage I, intermittent asthma: (I) symptoms <1/week; (II) transient symptoms; (III) nocturnal symptoms ≤2 times/month; (IV) FEV1% predicted ≥80%, or peak expiratory flow (PEF) ≥80% personal optimum value, or variation in PEF <20%; stage II, mild persistent asthma: (I) symptoms ≥1 time/week, <1 time/day; (II) may affect activity and morpheus; (III) nightly symptoms >2 times/month, <1 time/week; (IV) % predicted FEV1 ≥80%, or PEF ≥80% personal optimum value, or variation in PEF between 20% and 30%; stage III, moderate persistent asthma: (I) symptoms ≥1 time/day; (II) affects activity and morpheus; (III) nightly symptoms ≥1 time/week; (IV) % predicted FEV1 between 60% and 79%, or PEF between 60% and 79%, or variation in PEF ≥30%; stage IV, Severe persistent asthma: (I) symptoms ≥1 time/day; (II) frequent daytime and nocturnal symptoms; (III) any activity limitations; (IV) % predicted FEV1 <60%, or PEF <60% personal optimum value, or variation in PEF >30%.
Exclusion criteria were: (I) premature birth; (II) a history of any other lung disease except asthma; (III) the presence of serious systemic or chronic diseases, such as cardiorespiratory diseases, metabolic disorders, immunosuppression; (IV) symptoms of upper respiratory tract infection, for instance, rhinorrhea, fever and rhinobyon within 4 weeks, or used systemic corticosteroids within the prior 8 weeks; (V) difficulty in completing the questionnaires for any reason.
Pulmonary function tests
Pulmonary function was measured by the Jaeger Masterscope® spirometry system (Jaeger, Wuerzburg, Germany) according to an ATS/ERS statement (12). Three variables, forced vital capacity (FVC), FEV1, and FEV1/FVC, were derived from the best of three repeatable forced expirations.
FENO measurement
FENO was analyzed using a NioxMinor instrument (Aerocrine, Solna, Sweden) following the recommendations of the ATS/ERS (12) using the mean of two measurements. Measurements of FENO were performed before spirometry. Patients with high and low FENO level were defined by cut-off value of 25 ppb by ATS.
Asthma symptomatic control score
We used the Asthma Control Test (ACT) questionnaire to measure asthma control (26). The ACT score is a five-item questionnaire filled out by patients; activity limitation, shortness of breath, nighttime symptoms, use of rescue medication, and the patient’s overall scores for asthma control. Each item contains five corresponding answers options.
Statistical analysis
The Statistical Package for Social Sciences (SPSS), version 20.0 (IBM SPSS, Armonk, NY, USA), was used to analyze the data. Qualitative variables were expressed as number (%). Normally distributed data were expressed as mean ± standard deviation. The continuous variables were analyzed by Student’s t test, categorical variables were analyzed by chi-squared test.
Non-normally distributed data were expressed as median (inter-quartile range, IQR), and comparisons were made using the Mann-Whitney U-test. Logistic regression analyses were used to identify the variables associated with the outcome (asthma exacerbation). For all analyses, a P value of <0.05 was considered statistically significant.
Results
Baseline characteristics of the study subjects
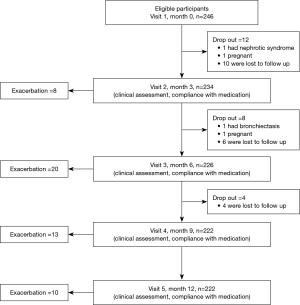
Two hundred and forty-six patients were randomly enrolled. Of these, 24 patients did not complete the study protocol (1 had bronchiectasis, 1 had nephrotic syndrome, 2 withdrew because of pregnancy, and 20 were lost to follow up). In total, 222 patients completed the study (Figure 1).
In 125 of the 222 patients (56.3%), the FENO level was elevated at baseline (>25 ppb). Patients had a median FENO level of 27 ppb (IQR: 10.0, 56.3 ppb), and the mean ACT score was 21 (range, 18–24). The mean FEV1% predicted was 80.7%±20.8%. The mean age of the patients was 40.7±12.7 years with a range of 14–65. Of the patients, 117 (52.7%) had good compliance. Forty-three (19.4%) of the patients used theophylline and 34 (15.3%) used leukotriene receptor antagonist. No patients used anti-IgE therapy. Baseline characteristics of the patients who completed the study are shown in Table 1.

Full table
Characteristics of patients with and without exacerbations
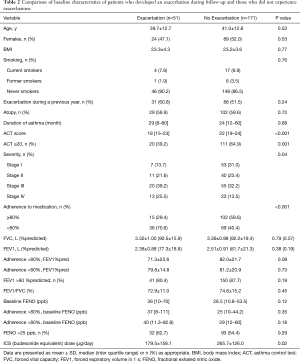
We compared demographic data between patients with and without asthma exacerbations (Table 2).
Full table
Fifty-one patients (23.0%) developed an exacerbation during the study period. Patient characteristics, including body mass index (BMI), age, sex, duration of asthma, smoking status, and atopy were not different between patients with and without exacerbations. Compared to patients with exacerbations, patients without an exacerbation had better treatment compliance (P<0.001). In the non-exacerbation group, the mean ACT score at baseline was higher (22, P<0.001) compared to the exacerbation group (18). Meanwhile, no significant differences were observed in pulmonary function indices, including FEV1% predicted, FVC% predicted, and FEVI/FVC, and FENO level between the two groups. After taking into account medication compliance, there were no statistical differences in FEV1% predicted and FENO between the two groups. However, there was a tendency of lower FEV1% predicted in patients with exacerbation (71.3±25.6 vs. 82.0±21.7, P=0.08). There were no differences for the number of exacerbations in the past 12 months between the two groups. More patients with exacerbations in the past 12 months had poor adherence with medications than those without exacerbations during the preceding year (61.0% vs. 39.0%, P=0.04, Table 3). Regardless of FEV1% predicted and FENO level, only about 50% of patients had good medication adherence.

Full table
Factors association with exacerbations
We used multivariate logistic regression analyses to identify the association between clinical parameters of baseline patients’ characteristics and asthma exacerbations.
Nonadherence to asthma medications was significantly associated with an increased risk of exacerbations (OR =3.548; 95% CI: 1.806–6.971; P<0.001). The OR remained high after adjustment for age, sex, BMI, ACT score, smoking status, severity, and the exacerbations in the past 12 months in addition to FEV1% predicted and FENO level (OR =4.718; 95% CI: 2.149–10.359; P<0.001; Table 4). Similarly, ACT score <20 was predictor of higher risk of exacerbations (OR =2.295, 95% CI: 1.130–4.663; P=0.022; Table 4). Meanwhile, the level of FENO, FEV1% predicted, severity, atopy, sex, age, BMI and smoking status were not associated with asthma exacerbations (date was not reflected in the article).

Full table
Discussion
To our knowledge, this is the first prospective 12 months follow up study to evaluate the risk factors of asthma exacerbations in real-world in China. The results showed that poor control of symptoms and nonadherence to asthma medication, rather than FEV1% predicted and FENO, increased risk of exacerbations in the study period.
The role of FENO and spirometry in predicting the future risk of exacerbations varied from studies, on the basis of the population characteristics and study design. Gelb et al. showed that combined measurement of FEV1% predicted and FENO might help stratify risk factors for future exacerbations of clinically treated asthmatics (20). Bjerregaard et al. showed that high FENO at baseline was risk factor for virus-induced exacerbations of patients with stable disease, while FEV1% predicted was not associated with increased risk of exacerbations (15). Tanaka et al. studied Japanese asthmatics with good adherence to treatment (>80%), and noted that FEV1% predicted was much higher in patients without exacerbation than in those with exacerbations, and also showed no difference in log-FENO between the two groups (6). There were no differences in pulmonary function indices and FENO between patients with and without exacerbations.
There are several possible reasons for the inconsistent results of studies with respect to pulmonary function indices and FENO. First, the current study was a non-interventional real-world study which included patients across the full clinical spectrum of the disease, such as different adherence to asthma medications. Compared to patients with asthma exacerbations, patients without exacerbations had better treatment compliance (P<0.001). After taking into account medication compliance, there were still no statistical differences in FEV1% predicted and FENO between patients with and without exacerbations. However, there was a tendency for lower FEV1% predicted in patients with exacerbation (P=0.08). Compared with previous research assessing the risk of future asthma exacerbations, the sample size of this study may have been relatively small (5,8,9). Therefore, if the sample size was much larger, there might exist statistical differences in FEV1% predicted and FENO between patients with and without exacerbations. Second, we did not categorize the degree of asthma exacerbations (mild/moderate/severe). As such, FEV1% predicted and FENO might have statistical significance in patients with different exacerbation severity. Third, in this study FEV1% predicted and FENO at baseline did not predict asthma exacerbation during the subsequent year. In a study with 3-year follow-up, Kimura et al. reported that FENO was a good predictor for exacerbations of severe asthmatics (16). In another prospective study, Kitch et al. reported that FEV1% predicted future exacerbations over 3-year intervals (8). The relatively short follow-up period of our study may partially explain the differing results as compared to other studies.
In this study, 47.3% of the patients ICS was <80% and 70.6% of patients who developed an exacerbation had poor adherence. Our findings are consistent with previously published studies that reported compliance ranges from 6% to 70% (27-31). Laforest et al. prospectively noted less than 25% of patients continuously using ICS over 1 year (29). Our study was limited to asthmatics lived in Guangzhou for more than 2 years, which may partly explain the higher medication compliance. And we provided interventions such as follow-up office visits, phone calls and text messages regularly to remind them that they are participating in the study. These patients may be more conscious of the potential severity of the disease and the necessity to take drugs as directed, leading to higher persistence rates. And our findings of adherence focus on the conception that medication corresponds to use, and patients subjectively evaluated their compliance, this means that discontinuation treatment might be even worse than reported. Corrao et al. performed a population-based study and showed that consistent use of ICS reduces the risk of exacerbations (32). Costa et al. demonstrated an association between poor adherence to medicine and exacerbation in asthmatic children (33). van der Merwe et al. performed a case-control study that demonstrated an association between reduced treatment adherence and severe life threatening asthma exacerbations (34). The results of the current study also demonstrated poor adherence to ICS was a significant risk factor of asthma exacerbation.
Many studies have reported the effectiveness of ACT in evaluating asthma control levels (35,36). Wei et al. and Ban et al. showed that current asthma control predicts future risk of asthma exacerbations (37,38). The current study clearly demonstrated that a lower ACT score was associated with a higher risk of asthma exacerbations.
It is known that a history of exacerbations during the preceding year may add the possibility of asthma exacerbations (5,6), but in the current study exacerbations during the preceding year were not different between patients with and without an exacerbation. This result may be due to adherence to asthma medication. More patients with exacerbations during the preceding year had poor adherence than patients without an exacerbation during the preceding year (61.0% vs. 39.0%, P=0.04). Additionally, we recorded exacerbations during the preceding year through a questionnaire, not with objective measures.
The study has several limitations. One limitation of this study is about the selection of the population. Since this is a single-center study, the studied population characteristics may be vary from the general population. Therefore, it is necessary to carry out larger multicentre studies. Another limitation is that there is no standardized approach to measure persistence rates, and each approach has its advantages and disadvantages (22,39). In the current study we simply stratified adherence of ICS as <80% and >80%. Also, if patients could not come to the respiratory outpatient clinic medication usage was determined by phone administration of questionnaires. In addition, we did not measure peripheral blood eosinophils, peak expiratory flow variation and airway reversibility. Besides, as a result of the variable nature of airway obstruction and inflammation with asthmatics, the fluctuation of FENO can’t justify a change in treatment. Finally, due to the lack of routine monitoring of asthmatics, such as FENO, PEF monitoring, spirometry, recording an exacerbation mainly relied on patients contacting the doctor when they had worsening and uncontrolled symptoms. It is possible the exacerbation rate may have been higher than recorded. However, we contacted patients regularly by phone to remind them that they are participating in the study. The advantage of this study is that it prospectively followed up patients with asthma in different population, which representing the daily clinical scenario experienced by most respiratory physicians.
In conclusion, in this non-interventional real-world study, baseline measurements of FEV1% predicted and FENO may not help to predict risk for future asthma exacerbations. Future multicenter studies are needed to confirm our results. Additionally, the present study showed that asthma exacerbations within the next 1 year were significantly more common in patients with poor symptom control and poor adherence to medications.
Acknowledgments
The authors would like to thank Professor Chunquan Ou of the Biostatistics Laboratory, School of Public Health, Southern Medical University, the patients and others who cooperated in performing this study.
Funding: This work was supported by the National Natural Science Foundation of China (81770033, 81470228, 81670026, 81700034), the Scientific and Technological Project of Guangdong Province (2016A020215117, 2017B020226006), the National Key Research and Development Plan of China (2016YFC0905800) and the Science and Technology Program of Guangzhou, China (201804010069).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of the hospital (code number NFEC2016-052), and all subjects have given their written informed consent prior to participation in the study.
References
- Taylor DR, Bateman ED, Boulet LP, et al. A new perspective on concepts of asthma severity and control. Eur Respir J 2008;32:545-54. [Crossref] [PubMed]
- Global Initiative for Asthma. Global strategy for asthma management and prevention: Updated 2018. Available online: http://ginasthma.org
- Haselkorn T, Fish JE, Zeiger RS, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol 2009;124:895-902.e1-4.
- Ernst P, Spitzer WO, Suissa S, et al. Risk of fatal and near-fatal asthma in relation to inhaled corticosteroid use. JAMA 1992;268:3462-4. [Crossref] [PubMed]
- Peters D, Chen C, Markson LE, et al. Using an asthma control questionnaire and administrative data to predict health-care utilization. Chest 2006;129:918-24. [Crossref] [PubMed]
- Tanaka A, Uno T, Sato H, et al. Predicting future risk of exacerbations in Japanese patients with adult asthma: A prospective 1-year follow up study. Allergol Int 2017;66:568-73. [Crossref] [PubMed]
- Fuhlbrigge AL, Kitch BT, Paltiel AD, et al. FEV1 is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol 2001;107:61-7. [Crossref] [PubMed]
- Kitch BT, Paltiel AD, Kuntz KM, et al. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest 2004;126:1875-82. [Crossref] [PubMed]
- Miller MK, Lee JH, Miller DP, et al. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med 2007;101:481-9. [Crossref] [PubMed]
- Kharitonov SA, Gonio F, Kelly C, et al. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J 2003;21:433-8. [Crossref] [PubMed]
- Kharitonov SA, Barnes PJ. Inhaled markers of pulmonary disease. Am J Respir Crit Care Med 2001;163:1693-722. [Crossref] [PubMed]
- American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. [Crossref] [PubMed]
- Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax 2003;58:175-82. [Crossref] [PubMed]
- Berry M, Hargadon B, Morgan A, et al. Alveolar nitric oxide in adults with asthma. Eur Respir J 2005;25:986-91. [Crossref] [PubMed]
- Bjerregaard A, Laing IA, Backer V, et al. High fractional exhaled nitric oxide and sputum eosinophils are associated with an increased risk of future virus-induced exacerbations: A prospective cohort study. Clin Exp Allergy 2017;47:1007-13. [Crossref] [PubMed]
- Kimura H, Konno S, Makita H, et al. Prospective predictors of exacerbation status in severe asthma over a 3-year follow-up. Clin Exp Allergy 2018;48:1137-46. [Crossref] [PubMed]
- Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FENO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 2015;70:115-20. [Crossref] [PubMed]
- Sivan Y, Gadish T, Fireman E, et al. The use of exhaled nitric oxide in the diagnosis of asthma in school children. J Pediatr 2009;155:211-6. [Crossref] [PubMed]
- Zeiger RS, Schatz M, Zhang F, et al. Elevated exhaled nitric oxide is a clinical indicator of future uncontrolled asthma in asthmatic patients on inhaled corticosteroids. J Allergy Clin Immunol 2011;128:412-4. [Crossref] [PubMed]
- Gelb AF, Flynn Taylor C, Shinar CM, et al. Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest 2006;129:1492-9. [Crossref] [PubMed]
- Global Initiative for Asthma. Global strategy for asthma management and prevention: Updated 2016. Available online: http://ginasthma.org
- Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007;10:3-12. [Crossref] [PubMed]
- Mann MC, Eliasson O, Patel K, et al. An evaluation of severity-modulated compliance with q.i.d. dosing of inhaled beclomethasone. Chest 1992;102:1342-6. [Crossref] [PubMed]
- Smith K, Warholak T, Armstrong E, et al. Evaluation of risk factors and health outcomes among persons with asthma. J Asthma 2009;46:234-7. [Crossref] [PubMed]
- Asthma Group of Chinese Thoracic Society. China Asthma Alliance. The Chinese experts' consensus on the evaluation and management of asthma exacerbation. Zhonghua Nei Ke Za Zhi 2018;57:4-14. [PubMed]
- Zhou X, Ding FM, Lin JT, et al. Validity of asthma control test for asthma control assessment in Chinese primary care settings. Chest 2009;135:904-10. [Crossref] [PubMed]
- Breekveldt-Postma NS, Koerselman J, Erkens JA, et al. Treatment with inhaled corticosteroids in asthma is too often discontinued. Pharmacoepidemiol Drug Saf 2008;17:411-22. [Crossref] [PubMed]
- Marceau C, Lemiere C, Berbiche D, et al. Persistence, adherence, and effectiveness of combination therapy among adult patients with asthma. J Allergy Clin Immunol 2006;118:574-81. [Crossref] [PubMed]
- Laforest L, Belhassen M, Devouassoux G, et al. Long-Term Inhaled Corticosteroid Adherence in Asthma Patients with Short-Term Adherence. J Allergy Clin Immunol Pract 2016;4:890-899.e2. [Crossref] [PubMed]
- Wu AC, Butler MG, Li L, et al. Primary adherence to controller medications for asthma is poor. Ann Am Thorac Soc 2015;12:161-6. [Crossref] [PubMed]
- Stoloff SW, Stempel DA, Meyer J, et al. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol 2004;113:245-51. [Crossref] [PubMed]
- Corrao G, Arfè A, Nicotra F, et al. Persistence with inhaled corticosteroids reduces the risk of exacerbation among adults with asthma: A real-world investigation. Respirology 2016;21:1034-40. [Crossref] [PubMed]
- Costa LDC, Camargos PAM, Brand PLP, et al. Asthma exacerbations in a subtropical area and the role of respiratory viruses: a crosssectional study. BMC Pulm Med 2018;18:109. [Crossref] [PubMed]
- van der Merwe L, de Klerk A, Kidd M, et al. Case-control study of severe life threatening asthma (SLTA) in a developing community. Thorax 2006;61:756-60. [Crossref] [PubMed]
- Koolen BB, Pijnenburg MW, Brackel HJ, et al. Comparing Global Initiative for Asthma (GINA) criteria with the Childhood Asthma Control Test (C-ACT) and Asthma Control Test (ACT). Eur Respir J 2011;38:561-6. [Crossref] [PubMed]
- Jia CE, Zhang HP, Lv Y, et al. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. J Allergy Clin Immunol 2013;131:695-703. [Crossref] [PubMed]
- Wei HH, Zhou T, Wang L, et al. Current asthma control predicts future risk of asthma exacerbation: A 12-month prospective cohort study. Chin Med J (Engl) 2012;125:2986-93. [PubMed]
- Ban GY, Ye YM, Lee Y, et al. Predictors of Asthma Control by Stepwise Treatment in Elderly Asthmatic Patients. J Korean Med Sci 2015;30:1042-7. [Crossref] [PubMed]
- Rand CS, Wise RA. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med 1994;149:S69-S76. [Crossref] [PubMed]