
Age at diagnosis is a heterogeneous factor for non-small cell lung cancer patients
Introduction
Lung cancer is the second most common malignant cancer and the leading cause of death from malignancy in the United States (1). Moreover, the prevalence of lung cancer has paralleled our increased life expectancy, with the median age at diagnosis 63 years (2). Elderly patients exhibit higher rates of mortality than younger patients with various solid cancers, regardless of the clinical characteristics of the primary tumor, including patients with advanced or metastatic non-small cell lung cancer (NSCLC) (3-5). In retrospective studies, younger lung cancer patients exhibited a higher incidence of adenocarcinoma, female, and an advanced stage disease (3,6). In addition, these patients tended to present with a higher malignant potential (7,8). Therefore, age is regarded as a heterogeneous fact for lung cancer patients. Interestingly, elder patients typically exhibit a lower frequency of lymph node metastasis, compared to patients with early-stage rectal cancer (9). The impact of age at diagnosis on clinicopathologic characteristics is not well known for lung cancer patients.
We hypothesize that the behavior of NSCLC will differ between patients diagnosed at different ages. In the present study, we sought to assess the association between age and lymph node metastasis, M stage, and overall survival (OS) by examining surveillance, epidemiology, and end results (SEER) Database.
Methods
SEER database and patient selection
Lung cancer patient records were obtained from the special SEER database from 2004 to 2013. The inclusion criteria were definitive NSCLC diagnosis by pathology; no radiotherapy prior to surgery; pathological TNM stage information; complete follow-up data after treatment. And the exclusion criteria are as follows: (I) patients with small cell lung cancer (ICD-0-3 histology code 8041–8045); (II) patients without pathologic diagnosis; (III) patients received neoadjuvant chemotherapy; (IV) patients without any TNM information; (V) patients with non-cancer specific death (cardiovascular related mortality, and other causes).
SEER*Stat Version 8.2.1 (2015; National Cancer Institute Cancer Statistics Branch, Bethesda, MD; www.seer.Cancer.gov/seerstat) was used to identify all patients with NSCLC based on the International Classification of Diseases for Oncology. Patients’ demographics on each case, including gender, age at diagnosis, extent of disease, primary site, histology, vital status, number of lymph nodes examined and positive, T stage, N stage, and M stage, were included. Adjuvant chemotherapy and neoadjuvant chemotherapy were not evaluated as the SEER registry does not include this information. The proportion of NSCLC patients was classified by 5-year intervals (0–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, and 85+). The primary endpoint of the study was calculated from the date of diagnosis to the date of cancer specific death or the last follow-up. The study was approved by institutional ethics committee of Shanghai Pulmonary Hospital (No. K16-264).
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Science (SPSS, Inc., Chicago, IL, USA) software, version 16.0 for Windows. The descriptive data were expressed as mean ± standard deviation. Baseline characteristics were analyzed by the chi-squared (χ2) test or Student’s t-test for continuous variables. The association between age at diagnosis and distant metastasis, and lymph node metastasis were evaluated by using Poisson regression. OS was displayed using Kaplan-Meier survival curves with 95% confidence intervals (CIs); the differences between curves were displayed using the log-rank test. All risk factors identified by univariate analysis were adopted in multivariate Cox proportional hazard analysis. A two-tailed test of a P value of ≤0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
In this retrospective study, 151,919 patients met the eligibility criteria. There were 72455 female patients (47.7%) and 79,464 male patients (52.3%). A total of 121,970 (80.3%) patients identified as white race. The median age at diagnosis was 68 years (range, 4–104 years). Of these patients, the 65–69 years old patient was also the greatest proportion (26,310, 17.3%), and 0–44 years old patient was the smallest proportion (3,134, 2.1%). As for treatment, 60,611 (39.9%) patients received radiation, and 49,862 (32.8%) patients received surgery. At follow-up, 54,004 (35.5%) patients were still alive. The 1-, 3-, and 5-year survival rates were 47.8%, 20.3%, and 10.3%. The median time of OS was 16.0 (95% CI: 15.813–16.187) months. According to AJCC TNM stage classification, 81,501 patients (53.6%) had at least one lymph node metastasis, 60,271 patients (39.7%) had M1 stage (including M1a and M1b). The greatest proportion was T4 stage (35,226 patients, 23.2% for T1 stage; 50,759 patients, 33.4% for T2 stage; 9,946 patients, 6.5% for T3 stage; 55,988 patients, 36.9% for T4 stage). In our cohort, 29.6%, 52.4%, 61.8%, and 68.5% of patients had stage T1, T2, T3, and T4 patients respectively, with lymph node positive, and 19.3%, 32.2%, 38.6%, and 59.5% for T1, T2, T3, and T4 patients with M1 stage. The proportion of NSCLC patients is presented in Table 1.
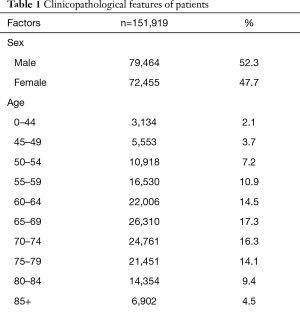
Full table
Age increases risks of lymph node metastasis and distant metastasis
As illustrated in Table 2, younger patients tended to have more node-positive disease, distant disease, adenocarcinoma, and black patients (P<0.001, respectively). Individuals younger than 45 years had a higher incidence of lymph node involvement (from 64.0% in patients 0–44 years old to 45.3% in patients 85 and over) and distant metastases (from 53.7% in patients 0–44 years old to 35.3% in patients 85 and over) (P<0.001, respectively) in each increasing age category (Figure 1A,B). And the percentage of population gradually increased by increasing age (Table 2 and Figure 1).
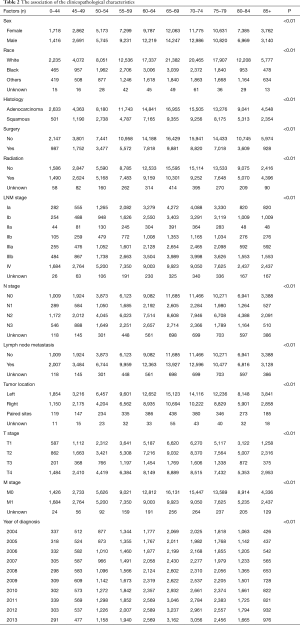
Full table
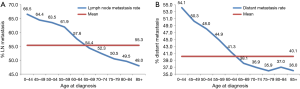
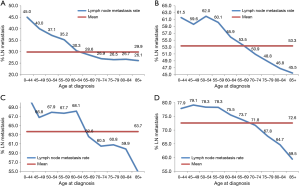
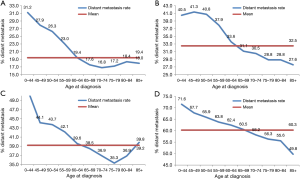
Subgroup analysis according to different T stages (T1, T2, T3, and T4 stage) showed that the risk of lymph node metastasis (Figure 2A,B,C,D) and M1 stage (Figure 3A,B,C,D) were also decreased regardless of T stage. The incidence of distant metastasis and lymph node metastasis gradually decreased with increasing age in different stages (P<0.001, respectively) (Figures 2 and 3).
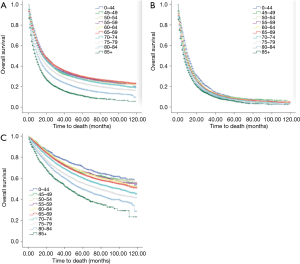
Age at diagnosis is an independent factor for predicting outcome
Age at diagnosis (Figure 4A,B,C), sex, race, year of diagnosis, histology, location of tumor, T stage, N stage, M stage, TNM, radiotherapy, and surgery (P<0.001, respectively) were statistically significant risk predictors for survival in the univariate analyses. In the multivariate analyses, age at diagnosis, sex, race, year of diagnosis, histology, location of tumor, T stage, N stage, M stage, TNM, radiotherapy, and surgery were analyzed as continuous or categorized variables and were significantly associated with an increased risk of death, independent of tumor location (Table 3).
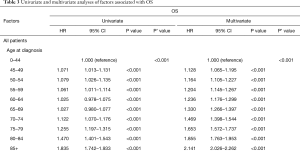
Full table
Discussion
In recent decades, the cancer-associated death rate in lung cancer patients has decreased. Improvements in medical treatment and increased public awareness have been considered to play a role. Previous studies showed that elderly NSCLC patients represent a heterogeneous group (10) with unfavorable clinicopathologic characteristics (11). For example, elderly patients have significantly more chemotherapy-related toxicity (12,13), and less possibility for adenocarcinoma histology compared with younger patients (4). However, the impact of age on clinicopathologic characteristics was assessed in small-scale populations, and there is still debate as to what this means for lung cancer patients. To address this, we assessed the effects of age on N stage and M stage using the SEER Database.
TNM stage is an independent prognostic factor for all solid cancers, including NSCLC (14,15). The relationship between the age at diagnosis and the risk of lymph node positivity in NSCLC has not been previous described. In this study, we demonstrated that younger patients diagnosed with NSCLC have an increased predisposition for lymph node positivity compared with older patients. The risk of metastasis in older patients is nearly double of the younger patients. We also assessed the incidence of distant metastasis in our cohort. The risk of distant metastasis followed a similar trend as that of lymph node metastasis in NSCLC patients. In order to further validate our results, patients were divided into four subgroups according to T stages. Remarkably, the inverse relationship between age and distant metastasis, and lymph node metastasis remained. These results support the idea that younger patients have a higher malignant potential compared to the elderly.
The relationship between age and survival is still controversial. In some small-scale studies, age as a continuous variable, was not a prognostic factor for advanced or metastatic NSCLC (10,16,17). Other studies have drawn the opposite conclusion that age is a prognostic factor for patients with NSCLC, and elderly patients have a worse outcome compared to younger individuals (3,18,19). In our current analysis, a large population-based study, we find that age is in fact a prognostic factor for this population (as assessed by the cancer-specific survival). We find an unexpected link between survival and lymph node metastasis. This is because younger patients may respond better to treatments, like surgery and chemoradiotherapy (20,21). Previous studies analyzed age as binary variable. In order to better assess the associations between age and clinicopathological characteristics, patients were classified by 5-year intervals.
Our data present an interesting phenomenon. However, we are unable to address the underlying mechanisms in our current study. One possible explanation for our results is a genetic difference between our patients. Tumor cells in younger patients may exhibit more aggressive behavior than those in older patients (22). Secondly, age-related changes are presented in immunologic surveillance, such as decreased lymphatic flow to nodes and/or nodal involution. It was reported that tumor-associated neutrophil is related to a metastatic disease (23,24), and age-related changes in natural killer cells can impact on their ability to perform immune surveillance (25). We must also consider that a higher proportion of elderly patients take aspirin regularly because of cardiovascular diseases, such as myocardial infarction (26,27), and coronary artery disease (28,29). Aspirin can inhibit the aggregation of platelet by influencing the activity of cyclooxygenase-1 (COX-1) (30). Platelets have been shown to promote tumor metastasis (31). Future studies will need to elucidate whether these variables support our findings.
Our study is inherently limited by its retrospective design. Although our study is based on a large population and multicenter analysis, some of the patients’ files have been miscoded. These clerical errors can lead to patients being excluded from the study. Additionally, some important information, such as details of treatment, surgery type and smoking status, were not available in the SEER Database. Previous studies reported that neoadjuvant chemotherapy increases pathological response and lymph nodal downstage, so it could reduce the incidence of lymph node metastasis (32). In this study, we excluded patients who received neoadjuvant radiotherapy to eliminate the effect of preoperative radiation on lymph node harvest and positivity. Another consideration is that incomplete lymph node dissections may lead to misdiagnosing a lymph node metastasis. Finally, the records were obtained from the SEER Database did not provide the sites of tumor invasion. Thus, it is not possible to accommodate the latest edition of TNM, and the T stage of these patients was defined by the 8th AJCC TNM staging classification (33).
In conclusion, age at diagnosis is a heterogeneous factor for NSCLC patients. This study demonstrates that young age is associated with increased rates of lymph node and distant metastases. However, in spite of this, we also find that age is an independent factor for predicting outcome: younger patients have a better prognosis.
Acknowledgments
Funding: This project is funded by the Shanghai Hospital Development Center (SHDC12015116).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee of Shanghai Pulmonary Hospital (No. K16-264).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Havlik RJ, Yancik R, Long S, et al. The National Institute on Aging and the National Cancer Institute SEER collaborative study on comorbidity and early diagnosis of cancer in the elderly. Cancer 1994;74:2101-6. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Rosen JE, et al. Lung Cancer in the Very Young: Treatment and Survival in the National Cancer Data Base. J Thorac Oncol 2016;11:1121-31. [Crossref] [PubMed]
- Kuo CW, Chen YM, Chao JY, et al. Non-small cell lung cancer in very young and very old patients. Chest 2000;117:354-7. [Crossref] [PubMed]
- Chen KY, Chang CH, Yu CJ, et al. Distribution according to histologic type and outcome by gender and age group in Taiwanese patients with lung carcinoma. Cancer 2005;103:2566-74. [Crossref] [PubMed]
- O'Brien KM, Sun J, Sandler DP, et al. Risk factors for young-onset invasive and in situ breast cancer. Cancer Causes Control 2015;26:1771-8. [Crossref] [PubMed]
- Ahrensberg JM, Fenger-Gron M, Vedsted P. Primary Care Use before Cancer Diagnosis in Adolescents and Young Adults - A Nationwide Register Study. PLoS One 2016;11:e0155933. [Crossref] [PubMed]
- Statius Muller MG, van Leeuwen PA, de Lange-De Klerk ES, et al. The sentinel lymph node status is an important factor for predicting clinical outcome in patients with Stage I or II cutaneous melanoma. Cancer 2001;91:2401-8. [Crossref] [PubMed]
- Pallis AG, Gridelli C. Is age a negative prognostic factor for the treatment of advanced/metastatic non-small-cell lung cancer? Cancer Treat Rev 2010;36:436-41. [Crossref] [PubMed]
- Meyer JE, Cohen SJ, Ruth KJ, et al. Young Age Increases Risk of Lymph Node Positivity in Early-Stage Rectal Cancer. J Natl Cancer Inst 2016.108. [PubMed]
- Sederholm C, Hillerdal G, Lamberg K, et al. Phase III trial of gemcitabine plus carboplatin versus single-agent gemcitabine in the treatment of locally advanced or metastatic non-small-cell lung cancer: the Swedish Lung Cancer Study Group. J Clin Oncol 2005;23:8380-8. [Crossref] [PubMed]
- Wheatley-Price P, Ding K, Seymour L, et al. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008;26:2350-7. [Crossref] [PubMed]
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Chen T, Zhang MG, Xu HX, et al. Preoperative serum CA125 levels predict the prognosis in hyperbilirubinemia patients with resectable pancreatic ductal adenocarcinoma. Medicine (Baltimore) 2015;94:e751. [Crossref] [PubMed]
- Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst 1980;65:25-32. [PubMed]
- Albain KS, Crowley JJ, LeBlanc M, et al. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 1991;9:1618-26. [Crossref] [PubMed]
- Ramalingam S, Pawlish K, Gadgeel S, et al. Lung cancer in young patients: analysis of a Surveillance, Epidemiology, and End Results database. J Clin Oncol 1998;16:651-7. [Crossref] [PubMed]
- Wu CY, Fu JY, Wu CF, et al. Survival Prediction Model Using Clinico-Pathologic Characteristics for Nonsmall Cell Lung Cancer Patients After Curative Resection. Medicine (Baltimore) 2015;94:e2013. [Crossref] [PubMed]
- Ramsey SD, Howlader N, Etzioni RD, et al. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results-Medicare. J Clin Oncol 2004;22:4971-8. [Crossref] [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [Crossref] [PubMed]
- Cote ML, Kardia SL, Wenzlaff AS, et al. Combinations of glutathione S-transferase genotypes and risk of early-onset lung cancer in Caucasians and African Americans: a population-based study. Carcinogenesis 2005;26:811-9. [Crossref] [PubMed]
- Granot Z, Henke E, Comen EA, et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011;20:300-14. [Crossref] [PubMed]
- Suzuki K, Kachala SS, Kadota K, et al. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res 2011;17:5247-56. [Crossref] [PubMed]
- Manser AR, Uhrberg M. Age-related changes in natural killer cell repertoires: impact on NK cell function and immune surveillance. Cancer Immunol Immunother 2016;65:417-26. [Crossref] [PubMed]
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1607-21. [Crossref] [PubMed]
- Meredith IT, Tanguay JF, Kereiakes DJ, et al. Diabetes Mellitus and Prevention of Late Myocardial Infarction After Coronary Stenting in the Randomized Dual Antiplatelet Therapy Study. Circulation 2016;133:1772-82. [Crossref] [PubMed]
- Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation 2006;113:203-12. [Crossref] [PubMed]
- Erglis A, Mintale I, Latkovskis G, et al. Management of coronary artery disease patients in Latvia compared with practice in Central-Eastern Europe and globally: analysis of the CLARIFY registry. Medicina (Kaunas) 2015;51:240-6. [Crossref] [PubMed]
- Li X, Fries S, Li R, et al. Differential impairment of aspirin-dependent platelet cyclooxygenase acetylation by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A 2014;111:16830-5. [Crossref] [PubMed]
- Yu LX, Yan L, Yang W, et al. Platelets promote tumour metastasis via interaction between TLR4 and tumour cell-released high-mobility group box1 protein. Nat Commun 2014;5:5256. [Crossref] [PubMed]
- Thomas M, Rube C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]


