
Endovascular treatment of complex diseases of the thoracic aorta—10 years single centre experience
Introduction
Pathological changes of the thoracic aorta occur in 5–6 cases per million people per year, with aortic aneurysm dissection and rupture being the most common (1). The prognosis of aortic dissection and rupture is poor, and mostly depends on the aortic level. Thus, dissections of the descending part of the aorta are associated with a 65–70% mortality within 4 years, uncomplicated type B dissections with a 30-day mortality of 3%, and a complicated dissection with a 25–50% mortality within 48 hours (1,2). Moreover, the type B complicated dissection, especially with an acute etiology has a very high likelihood of dying and requires emergent open surgical or stent graft treatment (1).
In the (International Registry of Acute Aortic Dissection) IRAD register the risk factors of descending aorta pathologies included: Age more than 60 years old, male sex, a pre-existing aortic disease or aortic valve, family history of aortic disease including colagenopathy, previous cardiac surgery, smoking, blunt chest trauma and intravenous drugs use (e.g., cocaine and amphetamines) (2,3).
For many decades, aortic aneurysms have been treated with open surgical procedures with outcomes comparable to conservative treatment, which is based solely on pharmacotherapy (4,5). There was a real breakthrough in treatment of descending aorta aneurysms in the early 1990s, by introducing the invasive endovascular techniques (5,6).
Interventional radiology is an essential part of modern operative treatment and fluoroscopically guided interventional procedures are increasingly performed during the past 15 years. In recent years, the numbers of thoracic stent graft placements increased (7,8). Endovascular repair of infra-renal thoracic aortic aneurysms (TEVAR) have become the generally accepted alternative to open surgery for selected patients and has been reported to reduce both morbidity and mortality (9,10). Pathologies of the aortic arch as well as its branches are more complex and require hybrid procedures: open vascular operation (aortic arch debranching techniques) enable safe stent graft deployment.
Nowadays, aortic diseases are treated by interdisciplinary teams that involves specialists in surgery, interventional radiology, anesthesiology and cardiology, as well as the use of sophisticated medical equipment (11,12). The main objective of our study is to present 10-year experience of aortic diseases treatment in a single centre with particular attention to efficiency and safety.
Methods
Patients
Between 2007 and 2017, 118 patients (38 females and 80 males) aged 21–85 years [mean age: 57.8 ± (SD) 13.4 years] with aortic arch and descending aorta pathologies were treated. There were 46 (39.0%) true aneurysms of the descending aorta and aortic arch, 68 (57.6%) Stanford type B aortic dissections, including 3 (2.5%) penetrating atherosclerotic ulcers with intramural hematoma, and 15 (12.7%) traumatic aortic aneurysms, 9 of them traumatic aortic ruptures. Other indications were; 1 (0.8%) Stanford type A aortic dissection, 1 stent dyshesion (0.8%) and 2 fistulas (1.7%). The baseline characteristics of all patients are presented in Table 1.
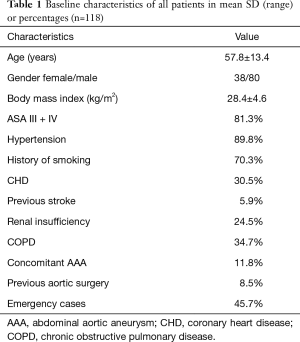
Full table
Informed consent from the patients was waived by the Local Bioethical Committee.
Stent graft implantation procedure
Among the 118 patients, thoracic stent grafting was performed in 98 patients in the endovascular room. Nine hybrid procedures were performed in two steps, first in the operating room and then in the vascular laboratory and another 11 hybrid procedures were performed in the hybrid room only. All procedures were done by a small team, consisting of two cardiac surgeons and one interventional radiologist. All but 2 procedures were performed with local anaesthesia, while 2 procedures were performed under general anaesthesia. Before surgery, all patients obligatory received a single shoot antibiotic prophylaxis and 5,000 IU of heparin, except in one case with a fracture of the skull base. Thoracic stent graft implantation was performed via the femoral approach. The left common femoral artery was punctured by Seldinger’s technique and the right common femoral artery was exposed in a surgical cut-down. A 6F straight catheter with side holes was introduced on a pigtail 5F into the ascending aorta. The prostheses were placed in the thoracic aorta over an Amplatz 0.35 guidewire. Finally, to examine the correct positioning and tightness of the prosthesis, DSA (Digital Subtraction Angiography) was performed. Generally, five to ten series were performed with the use of ionic contrast medium, depending on clinical indications. In patients who exceeded the dose of 1 Gy were additionally followed up for 6 months, and patients exceeding and 3 Gy were followed up for 1 year, with monthly visits and consultation of a dermatologist.
Hemi-arch transposition
Due to the inability to secure the stent graft implantation in LZ 1 (between brachiocephalic trunk and common carotid artery), it was decided to perform a two-step procedure. After a carotid-carotid anastomosis with Dacron vascular prosthesis 8 mm, a stent graft for the departure of the brachiocephalic trunk was implanted with covered the orifice of the left common carotid and left subclavian artery, in (Landing Zone) LZ 1.
Total-arch transposition
Due to the inability to secure the stent graft implantation in LZ 0, the patients were treated by a two-step procedure. Firstly, bifurcated Dacron 16/8 mm vascular prosthesis was interposed between the ascending aorta and brachiocephalic trunk and left common carotid artery from median sternotomy. During the same procedure or after 14 days, the stent graft was implanted into the ascending aorta, the proximal end of the distal to the anastomosis previously produced in ascending aorta, covering all the natural branches extending from the aortic arch and enlarged aneurysm sac (LZ 0). Eleven cases were performed during one procedure.
Postoperative evaluation
Patients after implantation of thoracic stent graft were being followed with examinations included computerized tomography at 1, 3, 6, 12 months after procedure, and later on once a year. Patients who had had the hybrid procedure were additionally followed-up every 3 months for the first 3 years.
Data management
Because of normal distribution the results are presented as mean ± SD, range or percentages.
Results
Stent grafts implanted
In 110 cases, thoracic aortic pathologies were excluded by Zenith stent grafts (Cook Inc., USA; Terumo Corp., Tokyo, Japan). In two patients Jotec (JOTEC GmbH, Stuttgart, Germany) stent grafts and in other six GORE (Gore Medical, Flagstaff, USA) stent grafts were implanted (one after Zenith because of type I endoleak). Prostheses with a diameter of 35.5±4.6 mm and length of 177.4±73.9 mm were implanted. Single stent grafts were used in 96 cases; two stents in 20 and 3 stent grafts in other two patients.

Full table
In our series, in 15 cases (LZ 0) the stent graft covered the ascending aorta, along with all the branches extending from the bow. In 4 cases (LZ 1), the prosthesis covered the common carotid artery. In 45 cases (LZ 2), the prosthesis covered the orifice of the left subclavian artery. In 54 patients (LZ 3 and 4), the proximal part of the stent graft was introduced just below the orifice of the left subclavian artery (see Table 2).
Among an overall of fifty-four emergent procedures, only 2 hybrid procedures were performed (hemi-arch transposition and anastomosis between the left common carotid artery and the left subclavian artery). The indications in 54 cases were: 34 acute dissections, 3 intramural hematomas, 15 post-traumatic acute thoracic aortic injuries and 1 stent graft dyshesion. In last patient, at the time of coronary angiography iatrogenic dissection of the ascending and descending aorta occurred. It was decided to implant a graft into the descending aorta below the left subclavian artery and the dissection of the ascending part of the aorta was treated with open surgery.
In 16 cases, graft implantation was performed urgently, in 13 patients the indication was symptomatic type B dissection, 1 patient had a false aneurysm; and in two cases fistulas after stent graft implantation. In one of those cases an arch debranching was performed. Elective surgery was performed in 48 patients, including 17 patients who underwent a two-step procedure. In 13 cases, a total-arch transposition was performed, consisting of suturing a bifurcated graft between the ascending aorta, brachiocephalic trunk, and the left common carotid artery and then implanting a graft covering all branches of the aortic arch. Three cases included concomitant treatment with hemi-arch transposition. In the first step, a cervico-cervical anastomosis was performed; then a stent graft was implanted, covering the left common carotid artery and the left subclavian artery. In 1 case the revascularisation of right subclavian artery was performed. In three cases, the indication was chronic B dissection of the descending aorta. Finally, in 45 cases, the indication was symptomatic aortic arch and descending aortic aneurysm (Table 2).
Early results
The technical success rate was 95.7%. One hundred and thirteen patients survived and were discharged. All periprocedural complications are included in Table 2. Six patients suffered periprocedural surgical complications. Retrograde dissection occurred in 2 patients. In 2 cases, the proximal part of the graft was implanted on the border of the orifice of the left carotid artery and it was necessary to implant a stent into carotid artery to preserve proper blood flow to the cerebral arteries. In 1 case brachial artery was punctured and the arteriovenous fistula was made requiting surgical treatment and in 1 case secondary to large atherosclerotic lesions in the femoral artery, after stent implantation there was lower limb ischemia which was resolved with angioplasty and stent implantation. Seven patients with thoracic aortic injury and 6 with type B dissection required hematoma evacuation from the left pleural cavity as a consequence of aortic pathology.
Sixteen patients presented medical complications. In 2 patients with aortic dissection, the renal arteries were supplied from a false channel. The stent graft implantation covered the false canal and caused the lack of flow to the kidneys. Other eight patients developed acute renal failure (contrast induced nephropathy) and needed continuous venovenous hemofiltration courses. One patient with history of peptic ulcer disease, gastrointestinal bleeding occurred despite the implementation of protons pump inhibitors, and there was one case of bowel necrosis that required urgent surgery and developed multi-organ dysfunction dying 33 days after stent graft implantation. Paraesthesia was developed in 2 patients (1.7%). In one case, the perioperative paraesthesia was probably associated with acute occlusion of the left subclavian artery that was treated medically. The clinical significance of this source of collateral perfusion of the spinal cord had not been confirmed previously. In this case, the symptoms resolved during hospitalisation. The other patient developed permanent paraesthesia because of spinal ischemia during complex procedure. Two patients suffered stroke (1.7%). Intracranial stroke was associated with prolonged manipulation of wires, catheters, and introducer sheaths within the aortic arch, reflected by a longer duration of the procedure.
In one case, the graft collapsed with a complete closure of the graft in the proximal segment at 3 months follow-up. The diameter of the aortic graft in the proximal segment increased from 37 mm before to 78 mm after procedure. An attempt to open the endograft endovascularly failed. The patient refused to undergo cardiac surgery and 6 years later he continued asymptomatic, without any further enlargement of the aortic diameter on control image studies.
Nine endoleaks were detected, 6 (5.1%) were primary (5 type I and 1 type II) and 3 (2.5%) secondary (1 type I and 2 type III). Secondary interventions were required in 8 (6.7%) of these patients. One of the reasons was type I endoleak treated by total-arch transposition and the necessity to reverse/retrograde dissection in the ascending aorta (A type) in 2 patients. Two patients with endoleak III were treated successful with another graft implantation and other 2 patients needed graft extension because of endoleak Ib. In one case after graft implantation because of oesophago-aortic fistula after previous graft implantation, 2 years later total arch debranching was performed to support type I endoleak. Fifty percent of patients experienced Velazquez syndrome (fever and leukocytosis—PIS—post implantation syndrome).
In the early postoperative period (within 30 days), five patients died. In one case, death resulted from acute type I endoleak and hypovolemic shock. Other causes included 2 ischaemic stroke and 2 multi-organ failure (one case after complex and multi-stage treatment of oesophago-aortic fistula). In the late period (12 months), other 5 patients died. All but one secondary to type I endoleak, due to multi-organ failure. The total mortality observed in our population was 8.5% (10 patients) (Table 3).
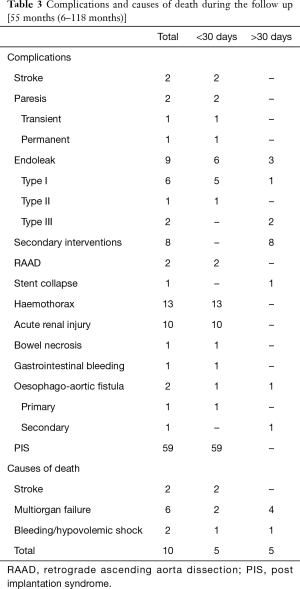
Full table
Discussion
Endovascular treatment is considered a major breakthrough in vascular surgery, being interventional radiology an essential part of modern surgical treatment of thoracic aortic diseases (8). Nowadays TEVAR has become a generally accepted alternative to open surgery, and has been reported to be effective in reducing both morbidity and mortality (8,9,13-23).
The most significant complications are retrograde type A dissection and endoleaks, especially types I and II. Erosion of the oesophagus or the left main bronchus is an extremely rare complication and potentially more related to the underlying disease than to the stent graft. TEVAR can induce neurological, brain, and spinal cord injury, and vascular complications (1-12).
A study published in 2010 examined patterns and mortality in TEVAR (22) and reported that in unruptured aneurysms the 30-day mortality for TEVAR was 5.2%, compared to 12% in open surgery. Patients having TEVAR, had a significant decrease in the length of hospitalization and intensive care unit (ICU) stay. Nevertheless, endovascular stent grafting appears to be safer for patients than traditional surgery, especially in high-risk patients with pre-existing severe neurological, cardiovascular, pulmonary, or renal dysfunction, by reducing duration of hospital admission and faster convalescence (13,24-26). A study by Gopaldas et al. (23) including 11,669 of endovascular repairs reported, that although TEVAR patients were 10 years older than the open repair cohort, and had a higher rate of comorbidities (peripheral vascular disease, chronic kidney disease, chronic pulmonary disease, and diabetes), the TEVAR technique was associated with a 61% lower overall complication. Despite the higher cost, TEVAR provided equivalent survival, earlier hospital discharge, and more frequent hospital discharge to home. In-hospital mortality was similar in both groups (2.3%), contrasting a previous report by Greenberg et al. (27) study, reporting significant higher 30-day mortality (5.7% vs. 8.3%, respectively, P=0.2, 12-month mortality (15.6% vs. 15.9%, P=0.9, and spinal cord ischemia complications (4.3% vs. 7.5%, P=0.08) between endovascular and open surgery treatment. Again, the endovascular cohort was older and sicker than the open surgical cohort.
Although the results of the surgical approach improved in the last decades, in-hospital mortality remains high, ranging between 25% and 50% (28). Complications associated with open surgery are: spinal cord ischemia (6.8%), stroke (9%), ischemia/mesenteric infarction (4.9%) and acute failure kidneys (19%) (29).
A European multicenter register of 50 patients reported a 30-day mortality rate of 8%, and an incidence of 8% of stroke and 2% of spinal cord ischemia (30). For Investigation of Stent Grafts in patients with Type B AD (INSTEAD), a total of 140 patients were randomized with subacute (>14 days) uncomplicated AD type B (31). A two-year observation showed that TEVAR is effective (aortic remodeling in 91.3% of patients treated with TEVAR vs. 19.4% in patients treated conservatively; P<0.001); however, no clinical benefits have been demonstrated with TEVAR compared to conservative treatment (interest survival: 88.9%±3.7% in the TEVAR group vs. 95.6%±2.5% in a group with optimal conservative treatment; P=0.15). The extended observation of this study (INSTEAD-XL) has been shown recently that mortality from aortic disease (6.9% vs. 19.3%, P=0.04) and disease progression (27.0% vs. 46.1%, P=0.04 respectively) were significantly lower after 5 years in the TEVAR treated group compared with only conservatively treated patients (32). No differences were found regarding total mortality. Similar observations described on the basis of the IRAD register. However IRAD also included patients with complicated AD, in that subgroup treated with TEVAR showed better 5-year survival compared with patients with medical therapy alone (29).
Available data indicates that TEVAR, in cases with appropriate anatomical conditions, it should be the preferred treatment option in TAI (2,3). In the review of 139 studies (7,768 patients), of which the majority were incomparable series of cases, retrospective research and none was randomized, a significantly lower mortality has been described in TEVAR than in open surgery (9% vs. 19%, P<0.01) (33).
Landing zones strategy and left subclavian artery orifice
In 2002, Mitchell et al. and Criado et al. introduced the division of the aorta into five LZ to indicate of localization stent graft and allow proper planning of, as necessary, a hybrid treatment (34,35). During the 2004 Tokyo (36) Consensus, guidelines have been formulated LZ, providing tight and stable placement of the proximal end of the prosthesis. The minimum length of fixation along the lesser curvature of the arch (aortic pathology-free) should be >20 mm. LZ aortic diameter >38/40 mm drastically increases the risk of leakage (endoleak I). Estimates of the radius of the curvature of the arc and the length of the aortic arch have a significant impact on the sustainability of graft fixation, mainly in LZ 2 and 3. Maximum oversizing of the prosthesis should not exceed 10% in TAA and should be avoided in type B dissection (13,37).
LZ 3 and 4 include the rest of the descending aorta. The location of the graft in LZ 3 and 4 does not require any additional vascular procedures. In 58 (73.4%) (LZ 3 and 4) patients, the proximal part of the stent graft was introduced just below the orifice of the left subclavian artery see and any other surgical procedure was needed.
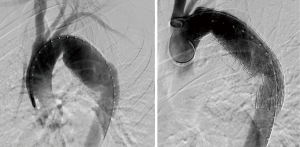
In cases where the position of the prosthesis by passing the left subclavian artery is not possible due to the lack of the correct length of the segment of the aorta, it is necessary to cover the mouth (LZ 2) (Figure 1). LZ 2 includes only part of the arch of the left subclavian artery. Based on many years of clinical observations have demonstrated that the procedure is safe. Vascular treatments restore circulation to the left subclavian artery are necessary only in 10–15% of cases in which the subclavian artery occlusion significantly interfere blood supply of the central nervous system (CNS) originating from the left vertebral artery. The absolute indications are diminutive, hypoplastic or absent right subclavian artery (RSA), left internal mammary artery coronary bypass graft, patient left axillary—femoral bypass graft, functioning left arm arteriovenous shunt for haemodialysis, aberrant origin and rare variants of the anatomical origin of the left vertebral artery (LVA) (14-18). In 45 (38.1%) our patients (LZ 2) cases, the prosthesis covered the orifice of the left subclavian artery without any vascular complications. The meta-analysis of Hajibandeh et al. proved that the routine LSA revascularization not reduce neurologic or mortality in patients undergoing TEVAR in LZ2 (38). Additionally Belczak et al. reported very low incidence of arm ischemia-related adverse events after LSA coverage without revascularization. The randomized trials are required to prove the routine revascularization strategy (39).
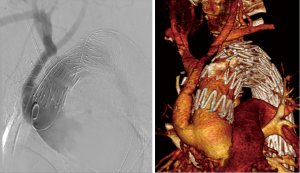
In cases where the pathology of the aortic arch the positioning of the prosthesis occupation forces LZ 0–1, only early partial debranching or debranching of the total aortic arch branches allows safe implantation of the proximal end of the vascular graft. When the proximal end of the graft is placed in LZ 1, it becomes equivalent to covering the orifice of the left common carotid artery and the left subclavian artery. In such cases, to maintain proper blood supply to the CNS, hemi-arch debranching must be performed, forcing at least revascularisation of the left common carotid artery. Vascular surgery routine in this case is a U-shaped bypass carotid-jugular prosthesis using Dacron with a diameter of 6–8 mm. If the graft implantation requires coverage of all branches of the aortic arch (LZ 0), the procedure will be safe when preceded by total-arch debranching (Figure 2). In the first stage of the procedure, aortic anastomosis is performed (bifurcated prosthesis of the ascending aorta) to the brachiocephalic trunk, the left common carotid artery, and possibly to the left subclavian artery. The second stage is endovascular thoracic stent implantation, covering the mouths of all branches of the arch. In our group 15 patients (12.7%) needed surgical procedures of total or hemiarch debranching. For patients safety becomes increasingly important meticulous plan the complete hybrid procedure, including taking decisions about one or two-step treatment. Each patient must be examined individually, often it is necessary to joint discussion cardiac surgeons, vascular surgeons and radiologists invasive.
Complications
Endovascular stent graft treatment is overall considered a safe and effective method, but is also associated with significant complications (19-21,27,28). The most common complications after stent graft implantation are endoleaks with and incidence ranging from 2.4% to 45.5% (40-42). To avoid endoleaks, the interventional procedure should be carefully planned, paying much attention on morphological details such as the length of the LZ, angulation and aortic calcification (porcelain aorta) according to Tokyo Consensus indications (34-36).
In our series, endoleaks occurred in 9 (7.6%) patients with TEVAR. In four of these patients, the proximal neck of the aneurysm was less than 2 cm long. The aneurysm of one of the patients with endoleak was enlarged by 7 mm in half a month. It was decided to execute transposition of the branches of the arch and an endograft was implanted in LZ 0. In 2 cases the type I endoleak (1 primary, 1 secondary) was the cause of hypovolemic shock and death. The secondary endoleaks type III in two cases were treated successfully with graft implantation.
Retrograde aortic dissection may occur after TEVAR. However, it is reported to be more frequent in acute aortic dissection and procedures, involving the aortic arch or the ascending aorta (24-26,43-47). Retrograde type A dissections are most commonly caused by endovascular prostheses with proximal bare springs (10,12,24,26), which are designed to provide proximal fixation of the stent graft. In the fragile aortic wall (14-21,46,47) the balloon dilatation is an additional risk of a further dissection. In our series, retrograde dissection occurred in one patient 10 days after the stent graft implantation.
Spinal cord ischemia after TEVAR is a severe, but rare complication with a reported incidence of 0.21%. The risk is correlated with extended lengths of the covered aortic segments. It is important to prevent the collateral blood supply via the left subclavian artery and lumbar arteries, as well as hypogastric arteries. The total length of the prosthesis covering the descending aorta longer than 205 mm was proved to increase the risk of spinal ischemia with consecutive paraplegia and additionally elevates the pressure of cerebrospinal fluid (3,47). In high-risk patients, the preventive cerebrospinal fluid (CSF) drainage is still recommended (has proven efficacy during open thoracoabdominal aneurysm surgery). Paraplegia progress can be stopped or even reversed by the interventional CSF drainage and pharmacological elevation of blood pressure (90 mmHg mean arterial pressure) (14,15,48-50). In our series, transient spinal cord ischemia occurred in one patient (1.2%) treated with thoracic stent graft. The symptoms of paresis occurred on postoperative day, and resolved during the 14 days of hospitalisation. The second patient developed permanent paraesthesia because of spinal ischemia during complicated procedure.
Arterial thrombosis, dissection, arteriovenous fistula, or pseudo aneurysm formation is reported in up to 3% of TEVAR procedures. To avoid these complications, the width and tortuosity of the femoral and iliac joints and the degree of calcification should be carefully evaluated by CT. In addition to appropriate surgical skills, it should be kept in mind that the introduction of the large-catheter system can induce vessel wall dissection or even perforation (51). In our study, arteriovenous fistula occurred in one patient after puncture of the axillary artery. It has been done in the open surgery way. In one case, stent implantation in the patient led to lower limb ischemia. Angioplasty with stent implantation was performed to restore proper flow of the peripheral arteries.
Closure of the graft is a rare complication; the incidence is estimated at 1.4–9.1% (52). In our work, one patient (1.2%) developed graft collapse. The main factor affecting the closure of the graft is excessive overvaluation, which can cause creasing of the graft and its subsequent susceptibility to the occurrence of complications (53,54). Other factors include the small diameter of the lumen, young age, and a small radius of the curvature of the aorta. At the same time, it has been suggested that a low force endograft expansion in graft proximal part cannot cope with challenging anatomic and haemodynamic conditions (high blood pressure or pulse) (52-54). For this reason, the balance between the ability to adapt to a flexible prosthesis and develop force should be one of the objectives in improving this type of treatment.
Brain injury complication of TEVAR can be associated with the excessive stent graft device manipulation within the arch, or overstenting of one or more of the great vessels (55-57). The left subclavian artery overstenting is permissible only in the emergency cases because of 10–15% risk of stroke and spinal cord injury (14,15,48-50,52-54). In our series, two patients (1.7%) had a stroke, in both it was the cause of death.
TEVAR has proved to be the treatment of choice of multi-organ hypoperfusion from dynamic true lumen compression. In some cases the additional branch vessel stenting may be necessary to prevent obstruction or false lumen thrombosis. The emergency interventional fenestration of the dissecting membrane is not considered (55,56).
Preoperative poor renal function, blood transfusions, contrast infusion and the thoracoabdominal extent of the aortic disease are the most important predictors for acute renal injury (ARI) (57,58). Patients with ARI experienced major postoperative complications, longer hospitalisation, and higher hospital mortality (58,59). 10 of our patients developed ARI and were treated with venovenous hemofiltration.
It is well known that haemothorax is an unusual but well-described complication of thoracic aortic dissection. In some cases, pleural effusion or haemothorax, rather than chest or back pain, is the first sign of aortic dissection (60). Emergency endovascular repair is recommended for patients who suffer from a large haemothorax or progressive increasing pleural effusion leading to respiratory dysfunction. In our series in 6 cases (7.5%) the haemothorax was the first symptom of aortic dissection and was present in 7 other cases of thoracic aorta injury. All patients needed delayed drainage during hospital stay.
Conclusions
Endovascular treatment for thoracic aortic diseases is an attractive alternative to open surgery and features significantly reduced morbidity and mortality compared to open surgical procedures. However, the effectiveness of TEVAR depends mainly on the experience and efficiency of the practitioner and careful selection of patients. Some patients with complex pathologies require hybrid procedures.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Local Bioethical Committee and informed consent from the patients was waived. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Svensson LG, Kouchoukos NT, Miller DC, et al. Expert Consensus Document on the Treatment of Descending Thoracic Aortic Disease Using Endovascular Stent-Grafts. Ann Thorac Surg 2008;85:S1-41. [Crossref] [PubMed]
- Moro H, Hayashi J, Sogawa M. Surgical management of the ruptured aortic arch. Ann Thorac Surg 1999;67:593-4. [PubMed]
- Buczkowski P, Puslecki M, Stefaniak S, et al. Post-traumatic acute thoracic aortic injury (TAI)—a single center experience. J Thorac Dis 2017;9:4477-85. [Crossref] [PubMed]
- Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002;35:1048-60. [Crossref] [PubMed]
- Zhou W, Reardon M, Peden EK, et al. Hybrid approach to complex thoracic aortic aneurysms in high-risk patients: surgical challenges and clinical outcomes. J Vasc Surg 2006;44:688-93. [Crossref] [PubMed]
- Brueck M, Heidt MC, Szente-Varga M, et al. Hybrid treatment for complex aortic problems combining surgery and stenting in the integrated operating theater. J Interv Cardiol 2006;19:539-43. [Crossref] [PubMed]
- Czerny M, Gottardi R, Zimpfer D, et al. Transposition of the supraaortic branches for extended endovascular arch repair. Eur J Cardiothorac Surg 2006;29:709-13. [Crossref] [PubMed]
- Greenhalgh RM, Brown LC, Kwong GP, et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomized controlled trial. Lancet 2004;364:843-8. [Crossref] [PubMed]
- Geijer H, Larzon T, Popek R, et al. Radiation exposure in stent-grafting of abdominal aortic aneurysms. Br J Radiol 2005;78:906-12. [Crossref] [PubMed]
- Zarins CK, White RA, Schwarten D, et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg 1999;29:292-305. [Crossref] [PubMed]
- Weigang E, Parker J, Czerny M, et al. Endovascular aortic arch repair after aortic arch de-branching. Ann Thorac Surg 2009;87:603-7. [Crossref] [PubMed]
- Gottardi R, Funovics M, Eggers N, et al. Supra-aortic transposition for combined vascular and endovascular repair of aortic arch pathology. Ann Thorac Surg 2008;86:1524-9. [Crossref] [PubMed]
- Puślecki M, Buczkowski P, Perek B, et al. Hybrid procedures for aortic arch repair. Kardiochi Torakochi Pol 2011;4:438-44..
- Chiesa R, Melissano G, Marrocco-Trischitta MM, et al. Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J Vasc Surg 2005;42:11-7. [Crossref] [PubMed]
- Gravereaux EC, Faries PL, Burks JA, et al. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. J Vasc Surg 2001;34:997-1003. [Crossref] [PubMed]
- Moore RD, Brandschwei F. Subclavian-to-carotid transposition and supracarotid endovascular stent graft placement for traumatic aortic disruption. Ann Vasc Surg 2001;15:563-6. [Crossref] [PubMed]
- Peterson BG, Eskandari MK, Gleason TG, et al. Utility of left subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg 2006;43:433-9. [Crossref] [PubMed]
- Riesenman PJ, Farber MA, Mendes RR, et al. Coverage of the left subclavian artery during thoracic endovascular aortic repair. J Vasc Surg 2007;45:90-4. [Crossref] [PubMed]
- Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 1994;331:1729-34. [Crossref] [PubMed]
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546-52. [Crossref] [PubMed]
- Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 1999;340:1539-45. [Crossref] [PubMed]
- Conrad MF, Ergul EA, Patel VI, et al. Management of diseases of the descending thoracic aorta in the endovascular era: a Medicare population study. Ann Surg 2010;252:603-10. [PubMed]
- Gopaldas RR, Huh J, Dao TK, et al. Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg 2010;140:1001-10. [Crossref] [PubMed]
- Umana JP, Mitchell RS. Endovascular treatment of aortic dissections and thoracic aortic aneurysms. Semin Vasc Surg 2000;13:290-8. [PubMed]
- Dake MD. Endovascular stent-graft management of thoracic aortic diseases. Eur J Radiol 2001;39:42-9. [Crossref] [PubMed]
- Brandt M, Hussel K, Walluscheck KP, et al. Stent-graft repair versus open surgery forthe descending aorta: a case-control study. J Endovasc Ther 2004;11:535-8. [Crossref] [PubMed]
- Greenberg RK, Lu Q, Roselli EE, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation 2008;118:808-17. [Crossref] [PubMed]
- Lansman SL, Hagl C, Fink D, et al. Acute type B aortic dissection: surgical therapy. Ann Thorac Surg 2002;74:S1833-5; discussion S1857-63.
- Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1:395-402. [Crossref] [PubMed]
- Heijmen RH, Thompson MM, Fattori R, et al. Valiant thoracic stent-graft deployed with the new captivia delivery system: procedural and 30-day results of the Valiant Captivia registry. J Endovasc Ther 2012;19:213-25. [Crossref] [PubMed]
- Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009;120:2519-28. [Crossref] [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [Crossref] [PubMed]
- Murad MH, Rizvi AZ, Malgor R, et al. Comparative effectiveness of the treatments for thoracic aortic transection (corrected). J Vasc Surg 2011;53:193-9.e1-e21.
- Criado FJ, Clark NS, Barnatan MF. Stent graft repair in the aortic arch and descending thoracic aorta: a 4-year experience. J Vasc Surg 2002;36:1121-8. [Crossref] [PubMed]
- Criado FJ, Abul-Khoudoud OR, Domer GS, et al. Endovascular repair of the thoracic aorta: lessons learned. Ann Thorac Surg 2005;80:857-63. [Crossref] [PubMed]
- Mitchell RS, Ishimaru S, Criado FJ, et al. Third International Summit on Thoracic Aortic Endografting: Lessons From Long-term Results of Thoracic Stent-Graft Repairs – Tokyo Consensus 2004 J Endovasc Ther 2005;12:89-97. [Crossref] [PubMed]
- 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases; available online: www.escardio.org/guidelines
- Hajibandeh S, Hajibandeh S, Antoniou SA, et al. Meta-analysis of left subclavian artery cowerage with and without revascularization in thoracic endovascular repair. J Endovasc Ther 2016;23:634-41. [Crossref] [PubMed]
- Belczak SQ, Silva ES, Klajner R, et al. Type II endoleaks, left-arm complications, and need of revascularization after left subclavian artey coverage for thoracic aortic aneurysm endovascular repair: a systematic review. Ann Vasc Surg 2017;41:294-9. [Crossref] [PubMed]
- Görich J, Rilinger N, Soldner J, et al. Endovascular repair of aortic aneurysm: treatment of complications. J Endovasc Surg 1999;6:136-46. [Crossref] [PubMed]
- Berg P, Kaufmann D, van Marrewijk CJ, et al. Spinal cord ischaemia after stent-graft treatment for infra-renal abdominal aortic aneurysms. Analysis of the Eurostar database. Eur J Vasc Endovasc Surg 2001;22:342-7. [Crossref] [PubMed]
- Maleux G, Koolen M, Heye S. Complications after endovascular aneurysm repair. Semin Intervent Radiol 2009;26:3-9. [Crossref] [PubMed]
- Mitchell RS, Ishimaru S, Ehrlich MP, et al. First International Summit on Thoracic Aortic Endografting: roundtable on thoracic aortic dissection as an indication for endografting. J Endovasc Ther 2002;9:II98-II105. [Crossref] [PubMed]
- Zhou W, Reardon ME, Peden EK, et al. Endovascular repair of a proximal aortic arch aneurysm: a novel approach of supra-aortic debranching with antegrade endograft deployment via an anterior thoracotomy approach. J Vasc Surg 2006;43:1045-8. [Crossref] [PubMed]
- Volodos’ NL, Shekhanin VE, Karpovich IP, et al. A self-fixing synthetic blood vessel endoprosthesis. Vestn Khir Im I I Grek 1986;137:123-5. [PubMed]
- Ma T, Dong ZH, Wang S, et al. Computational investigation of interaction between stent graft and aorta in retrograde type A dissection after thoracic endovascular aortic repair for type B aortic dissection. J Vasc Surg 2018;68:14S-21S.e2.
- Mosquera VX, Marini M, Fraga-Manteiga D, et al. Repair of Late Retrograde Type A Aortic Dissection After TEVAR: Causes and Management. J Card Surg 2016;31:164-7. [Crossref] [PubMed]
- Amabile P, Grisoli D, Giorgi R, et al. Incidence and determinants of spinal cord ischaemia in stent-graft repair of the thoracic aorta. Eur J Vasc Endovasc Surg 2008;35:455-61. [Crossref] [PubMed]
- Sullivan TM, Sundt TM 3rd. Complications of thoracic aortic endografts: spinal cord ischemia and stroke. J Vasc Surg 2006;43:85A-88A. [Crossref] [PubMed]
- Wan IY, Angelini GD, Bryan AJ, et al. Prevention of spinal cord ischaemia during descending thoracic and thoracoabdominal aortic surgery. Eur J Cardiothorac Surg 2001;19:203-13. [Crossref] [PubMed]
- Yusuf SW, Whitaker SC, Chuter TAM, et al. Early results of endovascular aortic aneurysm surgery with aortouniiliac graft, contralateral iliac occlusion, and femorofemoral bypass. J Vasc Surg 1997;25:165-72. [Crossref] [PubMed]
- Canaud L, Alric P, Desgranges P, et al. Factors favoring stent-graft collapse after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg 2010;139:1153-7. [Crossref] [PubMed]
- Canaud L, Hireche K, Berthet JP, et al. Endovascular repair of aortic arch lesions in high-risk patients or after previous aortic surgery: Midterm results. J Thorac Cardiovasc Surg 2010;140:52-8. [Crossref] [PubMed]
- Steinbauer MGM, Stehr A, Pfister K, et al. Endovascular repair of proximal endograft collapse after treatment for thoracic aortic disease. J Vasc Surg 2006;43:609-612. [Crossref] [PubMed]
- Kotelis D, Bischoff MS, Jobst B, et al. Morphological risk factors of stroke during thoracic endovascular aortic repair. Langenbecks Arch Surg 2012;397:1267-73. [Crossref] [PubMed]
- Melissano G, Tshomba Y, Bertoglio L, Rinaldi E, Chiesa R. Analysis of stroke after TEVAR involving the aortic arch. Eur J Vasc Endovasc Surg 2012;43:269-75. [Crossref] [PubMed]
- Patel HJ, Shillingford MS, Williams DM, et al. Survival benefit of endovascular descending thoracic aortic repair for the high-risk patient. Ann Thorac Surg 2007;83:1628-33. [Crossref] [PubMed]
- Azizzadeh A, Sanchez LA, Miller CC, et al. Glomerular filtration rate is a predictor of mortality after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2006;43:14-8. [Crossref] [PubMed]
- Khoynezhad A, Donayre CE, Smith J, et al. Risk factors for early and late mortality after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg 2008;135:1103-9. [Crossref] [PubMed]
- Rapezzi C, Longhi S, Graziosi M, et al. Risk factors for diagnostic delay in acute aortic dissection. Am J Cardiol 2008;102:1399-406. [Crossref] [PubMed]


