
Feedback on the use of three surgical sealants for preventing prolonged air leak after robot-assisted anatomical lung resection
Introduction
Anatomical lung resection is the standard treatment for early-stage lung cancer. The standard procedure consists in pulmonary lobectomy. Sub-lobar resections such as lung segmentectomy represent alternatives to lobectomy for patients with tumors <2 cm, patients with impaired lung function and for elderly patients (1). Pulmonary resections can be performed either with open or minimally invasive surgery, known as video-assisted thoracic surgery (VATS). VATS is associated with fewer complications and shorter length of hospital stay compared to open thoracotomy (2). More recently robotic assistance has emerged in thoracic surgery. Robot-assisted thoracic surgery (RATS) could help overcome the difficulties associated with VATS by reproducing the qualities of open surgery in a minimally invasive environment, bringing 3D vision and movements with 7 degrees of freedom as the human wrist (3).
Prolonged air leak (PAL) is the most common complication after pulmonary resection, occurring in 8% to 26% of patients and refers to any leakage of air from the lung that persists beyond the postoperative period (4). PAL often resolves without intervention, but results in an increase in chest drain duration and length of stay. Moreover there is an increased risk of complications like empyema, as well as costs (5,6).
Several products have been developed to seal the lung in order to reduce PAL occurrence. Fibrin-based sealants (FS) are two-component materials consisting of fibrinogen and thrombin presented as hemostatic, bioadhesive and sealant agents (7). Collagen-fleece-bound FS demonstrated efficiency in PAL prevention after lung resection performed through thoracotomy (8-11). Some synthetic sealants are composed of a polymeric material able to polymerize in the presence of a biological product, such as polyethylene-glycol (PEG). Biodegradable PEG-based sealant (PEGS) formed by extemporaneous mixing of a PEG-based crosslinker functionalized with succinate groups and human serum albumin demonstrated benefits in preventing PAL after open or minimally invasive lung resection (12,13). Other synthetic sealants are composed of materials able to polymerize in absence of biological products, such as polyglycolic acid-based sealants (PGAS). PGA felts have been proposed to reduce the incidence of PAL (14,15).
Different kinds of surgical sealants have been proposed to prevent the occurrence of PAL after lung resection surgery. However their benefits are not obvious and some authors do not recommend their routine use (16,17). In addition, few studies have reported their use after minimally-invasive thoracic surgery and no studies specifically after RATS lung resection. Thus in this work we aim to report our use of surgical sealants for preventing PAL after RATS lung resection.
Methods
Study design
A single center retrospective study was conducted, including patient who had robot-assisted mini-invasive lobectomy or segmentectomy between January 2012 and April 2018. Exclusion criteria were non-anatomical resection (wedge resection) and the occurrence of intraoperative complications requiring conversion to thoracotomy. Patients with a surgical sealant other than the three described were also excluded from this study. PAL was defined as an air leak lasting beyond postoperative day 5. The study obtained ethics approval provided by the institutional ethics committee (CERDE-HLJ: Comité d’Ethique pour la Recherche sur Données Existantes et/ou Hors Loi Jardé) with the number E2019-17.
Data collection
Data were collected by the surgeon using the French prospective thoracic surgery database EPITHOR and controlled by the data manager of this database. Patient consent was obtained for inclusion in the database, with each patient informed that the data would be used for research purposes. This collection was then double-checked by the analysis of computerized files (CDP2® software: CPage Patient File 2, version 7.7.2, Bourgogne Study Center of Hospital Information Systems, Dijon, France).
Demographic data [age, sex, weight, height, body mass index (BMI) and comorbidities] were extracted from the EPITHOR database. American Society of Anesthesiologists (ASA) preoperative physical status was also recorded.
Pre-operative PAL risk was evaluated according to the IPAL score developed using the EPITHOR database in case of VATS. This score is based on six variables: gender, dyspnea score, type of lung resection, the location of resection, pleural adhesion and BMI). A patient who presents an IPAL <5% has a low risk of PAL after pulmonary resection. With 5%< IPAL <6.9%, the risk of PAL is moderate and with IPAL >7% the risk of PAL is high.
Data relative to surgery (date, type of resection, operating time, and operative complications), chest drain duration, length of stay and postoperative complications classified according to Clavien-Dindo (18) were also collected using the EPITHOR database.
The number and type of surgical sealants were collected retrospectively by consultation of traceability sheets that were systematically completed during surgery. This collection was then double-checked using hospital pharmacy traceability and inventory management software (PHARMA® software version 5.8.70927.1400, Computer Engineering, Paris, France).
Surgical technique
A modified 3-arm technique was employed using the da Vinci Si® robot (Intuitive Surgical, Sunnyvale, CA, USA) (19). Three reusable trocars were used (12 mm for the camera, 8.5 mm for instruments). A single use trans-diaphragmatic assistant trocar placed in the 9th/10th intercostal space allowed access to suction and stapling supplies. Carbon dioxide insufflation (5–8 mmHg) facilitated dissection and improved tissue exposure. Dissection was performed with an EndoWrist® Maryland Bipolar Forceps (Intuitive Surgical) and an EndoWrist® grip (thoracic grasper or fenestrated bipolar forceps, Intuitive Surgical). Bronchovascular section was carried out by stapling, performed by a trained surgical resident. An assistant trocar incision was enlarged at the end of the procedure to remove the surgical specimen in an endoscopic retrieval bag. Pleurodesis was never performed immediately after surgery. When it occurred, it was for surgical revision away from resection surgery.
Surgical sealants
TachoSil® (Nycomed, Linz, Austria) is a collagen patch coated with human fibrinogen and thrombin (FS), approved in hemostasis improvement, tissue adhesion promotion and suture strengthening. The patch can be cut to the appropriate size and shape, so that it extends 1 to 2 centimeters beyond the wound margin. To facilitate passage through the thoracic cage, FS can be rolled up in a compress and then immobilized in place during 3 minutes with a pre-moistened compress.
Progel® (Bard Davol, Warwick, NY, USA) is a PEGS formed by extemporaneous mixing of a polyethylene glycol-based (PEGS) crosslinker functionalized with succinate groups and human serum albumin, marketed as a medical device and indicated in the prevention of PAL. PEGS is presented as a kit with two syringes, one containing human serum albumin and the other containing PEG crosslinker. After extemporaneous mixing of the two components, an elastic gel is applied on the tissue site. Two tips may be used. A steam tip allows application on a well-defined area such as staple lines. A spray tip allows coverage of a large area such as dissection areas. Extended spray tips (16 or 29 cm) may be used to reach distant area.
Neoveil® (Gunze, Osaka, Japan), a felt composed of polyglycolic acid, is a PGAS indicated in the reinforcement of sutured areas and in the prevention of air leaks. PGAS is available in a sheet type or a tube sheet. The sheet type is the only one used in our department.
Surgical sealant was applied on the area considered as potentially at risk of air leakage by the surgeon. This area may be staple lines, hilar dissection area or other dissection areas.
The choice of the surgical sealant was made according to surgeon experience and the availability of the product in the operating room.
Statistical analyses
Categorical data were reported using number and percentage. Continuous variables were expressed as median and 25–75% interquartile range. The exact Fisher’s test was used to compare categorical data. The non-parametric Wilcoxon-Mann-Whitney test was used to compare continuous variables except for the comparison of patients according to surgical sealant, which was performed with the non-parametric Kruskal-Wallis test.
A power analysis was performed. The expected PAL rate for RATS was 14.38% without sealant (20). The sealant was expected to divide by two the risk of PAL (14.38% → 7.19%). Considering a two-sided type I error rate at 5% and 299 subjects, the power was estimated at 81%, based on a chi-square test.
Unadjusted and adjusted logistic regression analyses were conducted. Adjustment variables were potentially major confounding defined a priori: IPAL and ASA score. Both adjustments were linear (quantitative variables). A sensitivity analysis adjusted on IPAL and ASA score and excluding patients operated in 2012 was performed.
P values <0.05 were considered statistically significant. Statistical analyses were performed using R software, version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
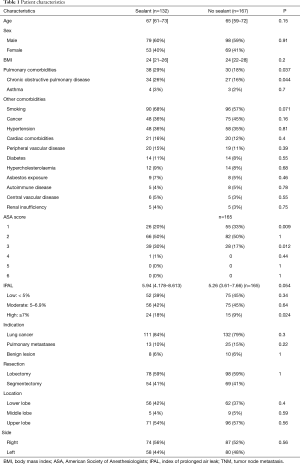
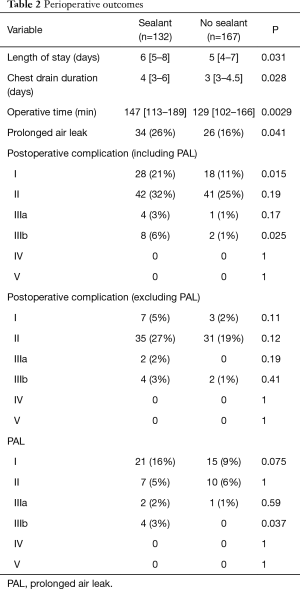
Between January 2012 and April 2018, 317 patients had RATS lobectomy or segmentectomy. Eighteen patients were excluded from the study because conversion to thoracotomy was required. Of the 299 patients included in the study, PAL occurred in 60 (20%). A surgical sealant was used at the end of the surgical procedure for 132 patients (44%) (Table 1). Regarding patient characteristics, age, sex, BMI, indication, extension and localization of surgery, were similar in patients with a sealant and in patients with no sealant. Pulmonary comorbidities were significantly higher in patients with a sealant (29% vs. 18%, P=0.037), especially there were more patients suffering from chronic obstructive pulmonary disease (26% vs. 16%, P=0.044). Other comorbidity rates were similar. There were significantly more ASA score 3 (30% vs. 17%, P=0.012) and IPAL ≥7% (18% vs. 9%, P=0.024) in patients with a sealant than in patients with no sealant who more often had ASA score 1 (33% vs. 20%, P=0.009). Length of stay, chest drain duration and operative time were significantly longer and PAL was more frequent in patients with a sealant (26% vs. 16%, P=0.041) (Table 2).
Full table
Full table
This result was confirmed by unadjusted multivariate analysis: odds ratio =1.88 (95% CI: 1.07 to 3.36, P=0.03). In contrast, when the analysis was adjusted to IPAL and ASA score, PAL risk was not modified by the use of a sealant: odds ratio =1.70 (95% CI: 0.94 to 3.10; P=0.08). There were significantly more grade I (P=0.015) and grade IIIb (P=0.025) complications in patients with a sealant. These 4 grade IIIb complications were all surgical revisions for pleurodesis. Excluding PAL, complication rates were not different between the groups.
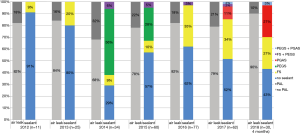
Three different surgical sealants were used during the study period (Figure 1). FS was used throughout the period; PEGS was used from 2014 to 2016 and PGAS from 2017 to the end of the study period. In 2012, no sealant was used in most cases (91%). This proportion decreased to 43% in 2018. Excluding the surgeon initial learning curve, e.g., patients operated in 2012, the risk of PAL was also not modified by the use of a sealant: odds ratio =1.74 (95% CI: 0.95 to 3.23; P=0.07).
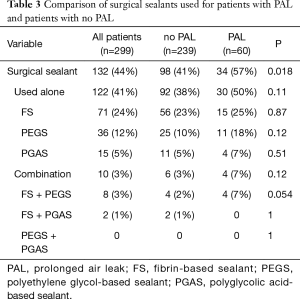
The description of the surgical sealant used alone or in combination is presented in Table 3. Among patients with PAL, 57% received a surgical sealant compared to 41% of patients with no PAL (P=0.018). Regarding surgical sealants, no significant difference was observed whether they were used alone or in combination.
Full table
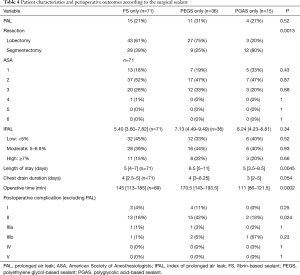
After excluding 10 patients who received a combination of two surgical sealants, patients were grouped according to the sealant that was used during RATS surgery (Table 4). PAL occurrence, ASA score, IPAL, and chest drain duration were not significantly different between groups. More segmentectomies were performed in the PGAS group than in the FS and PEGS groups (80%, 39% and 25% respectively, P=0.0013). Length of stay was 6.5 days in the PEGS group and 5 days in the FS and PGAS groups (P=0.0045). Operative time was respectively 145, 170.5, 111 min in the FS, PEGS and PGAS groups (P=0.0002). Excluding PAL, more grade II complications were observed in the PEGS group (P=0.024).
Full table
Discussion
To our knowledge this study is the first to report the use of surgical sealants specifically after RATS while the use of robotic assistance in thoracic surgery is growing (21). Several prospective or retrospective studies have investigated their use after open thoracic surgery (22). A single prospective study evaluated PEGS after minimally invasive surgery, in a cohort including 72 RATS and 40 VATS (13).
We observed a PAL incidence of 20%. This rate is consistent with the RATS associated literature. Indeed authors reported rates from 5.2% to 25.42% (pooled result: 14.38%) (20). We set 5 days post-operatively as the threshold to define PAL. This threshold is variable among published studies, from 4 days postoperatively to 10 days postoperatively. Since recent studies have demonstrated an average length of stay of 5 days after lobectomy, this 5-day threshold represents a duration which exceeds the average length of stay for lobectomy (4,23).
In randomized prospective studies, intraoperative air leak evaluation is performed prior to “selective” use of a sealant. Selective use consists in highlighting the presence and localization of air leakage by immersing the lung in saline or water and then reinflating it. An observation of air bubbles, followed or not by the calculation of a score (24) defines the presence of an intraoperative air leak. On the contrary, routine use consists in systematically applying surgical sealant at the end of the procedure. In our study, air leakage was not highlighted by a lung immersion test. Indeed, it may be sometimes difficult to detect air leaks in a chest cavity in which the lung is returned to the chest wall. Surgical sealants are not used routinely in our department but their use is based on surgeon experience and on surgery context.
Moreover the longer operative time observed for patients with a sealant could be associated with per-operative difficulties. Extended pleural symphyses and incomplete fissures are frequently encountered and may be responsible for important parenchymal breaches. Surgeons therefore tend to use a sealant to limit the risk of air leakage. An inverse trend was observed in the PGAS group. One possible explanation is the higher proportion of segmentectomies in this group. Operative times reported in the literature are indeed lower for segmentectomies than for lobectomies (25,26).
In this study, we were not able to demonstrate any benefit of using surgical sealants to prevent PAL. Many surgical sealants for preventing PAL have been described in the literature and some of them showed an interest both in-vivo and for clinical use. A Cochrane systematic review including 16 trials concluded that surgical sealants used selectively may reduce PAL and chest drain duration (22). Despite these results, the benefits of surgical sealants remain controversial. A survey published in 2009 showed that 15% of thoracic surgeons were skeptical about the usefulness of surgical sealants. The authors also highlighted that sealants were sometimes misused and poorly known by thoracic surgeons. Indeed, 20% of thoracic surgeons do not know the mode of action of the glues they use (27).
The problem of air leakage has motivated innovation in the field of surgical sealants. Three different sealants were mostly used in our center during this 7-year period. Other sealants have been used intermittently such as BioGlue® (Cryolife, Kennesaw, Georgia, USA), Tridyne® (Bard Davol, Warwick, NY, USA) and Surgicel® (Johnson & Johnson, New Brunswick, New Jersey, USA). Only seven RATS patients were concerned by these sealants and they were not included in this study. The most used sealant in this study was FS. Other authors have identified FS as the most used sealant (27). TachoSil® was approved in Europe in 2004, by the FDA in 2010 and is indicated to improve hemostasis, promote tissue adhesion and strengthen sutures. In our establishment, FS is used in these indications by several surgical disciplines such as cardiovascular, digestive, gynecological, ENT and urologic surgery. FS has been used for the prevention of leaks in thoracic surgery since 2012 and is still used. PEGS and PGAS are reserved for thoracic surgery. Progel® was approved by the FDA in 2010 and received the European Union CE mark in 2011. In our center PEGS was first ordered at the beginning of 2014. Neoveil® received the CE mark in 2012 and was approved by the FDA in 2013. PGAS is typically used on the lung in combination with a fibrin glue to adhere the sheet (15). Since its introduction in our thoracic surgery department in 2017, we do not use fibrin glue to adhere the patch. Instead of fibrin glue, a “blood patch” is used. A compress soaked with the patient’s blood is applied to the PGAS sheet after parenchymal stapling. This technique has been described to promote pleural symphysis for other interventions than lung resection (28). This product and this non-typical use are still being evaluated in our surgery department.
The choice of the surgical sealant used was made according to the availability of the products in the operating room and according to the surgery context. The surgeon favored the use of PGAS for stapling dense parenchymal areas and emphysematous lungs, which are at high risk of air leakage. In a context of extended pleural symphysis, the spray application of PEGS allows coverage of a large parenchyma area. It can be used to strengthen bronchial or parenchymal staple lines. The primary indication for FS is homeostasis. It can also be used for aerostatic purposes; however its application may sometimes be difficult because of its friable composition. Its size also limits the extent of aerostatic coverage, as well as all patch presentations compared to aerostatic gels. In contrast, the mechanical properties of the patch are more effective for localized air leakage in an emphysematous zone. Moreover patches are self-maintained between the lung and the pleural cavity. Currently, another PEG-coated collagen patch is being tested in our thoracic surgery department (Hemopatch®, Baxter AG, Vienna, Austria).
We observed more grade II complications when the sealant used was PEGS, as arrhythmias, urinary retentions, pneumonitis, digestive complications and atelectasia. In our opinion, these complications do not appear to be attributable to PEGS. Indeed adverse events reported in a pivotal clinical trial were adverse events that normally occur after pulmonary resection, as fever, nausea, confusion, constipation, and dyspnea. More pneumonia occurred in the control group and no difference was observed in the incidence of other adverse events, including atrial fibrillation (12).
This study has several limitations. It is a single center retrospective study even though parts of our data were collected from the national database of the French Society of Thoracic and Cardiovascular Surgery (EPITHOR). A multicenter study would have mitigated the effects of local practices, such as blood-patch for adhesion of PGAS. All surgical procedures were performed by two surgeons, one of whom performed nearly 90% of procedures. Thus, the question of the learning curve arises especially since the year 2012, the beginning of the study period, which corresponds to the implementation of the technique in our establishment, even if both surgeons were experienced in minimally invasive surgery and had specific training for RATS. Moreover a sensitivity analysis excluding the year 2012 did not show any difference. The decision to apply a surgical sealant was subjective, based on the experience of the surgeon and the surgical context, as well as where it was applied. A more rigorous study with a selective use of surgical sealant would conclude with more certainty the effectiveness or not of surgical sealants in patients who are objectively at risk of developing PAL.
Conclusions
In this retrospective study we report our experience of the use of surgical sealants after RATS over a 7-year period. We were not able to demonstrate any benefit of using a surgical sealant for preventing PAL, despite the variety of products used. One likely explanation is highlighted in this real-life study. The methods for choosing whether or not to apply a surgical sealant as well as its location were based on surgical context. Surgical sealants were thus used for the most at-risk patients. However surgical practices in our center could be improved. In patients the most at risk of air leakage, a lung immersion test should be performed. This would help to rationalize the use of surgical sealants for preventing PALs.
Acknowledgments
The authors are grateful to Nikki Sabourin-Gibbs for her help in editing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study obtained ethics approval provided by the institutional ethics committee (CERDE-HLJ: Comité d’Ethique pour la Recherche sur Données Existantes et/ou Hors Loi Jardé) with the number E2019-17. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Singhal S, Ferraris VA, Bridges CR, et al. Management of alveolar air leaks after pulmonary resection. Ann Thorac Surg 2010;89:1327-35. [Crossref] [PubMed]
- Brunelli A, Xiume F, Al Refai M, et al. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest 2006;130:1150-6. [Crossref] [PubMed]
- Varela G, Jimenez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [Crossref] [PubMed]
- Spotnitz WD. Fibrin Sealant: The Only Approved Hemostat, Sealant, and Adhesive-a Laboratory and Clinical Perspective. ISRN Surg 2014;2014:203943. [Crossref] [PubMed]
- Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg 2007;31:198-202. [Crossref] [PubMed]
- Droghetti A, Schiavini A, Muriana P, et al. A prospective randomized trial comparing completion technique of fissures for lobectomy: stapler versus precision dissection and sealant. J Thorac Cardiovasc Surg 2008;136:383-91. [Crossref] [PubMed]
- Marta GM, Facciolo F, Ladegaard L, et al. Efficacy and safety of TachoSil(R) versus standard treatment of air leakage after pulmonary lobectomy. Eur J Cardiothorac Surg 2010;38:683-9. [Crossref] [PubMed]
- Rena O, Papalia E, Mineo TC, et al. Air-leak management after upper lobectomy in patients with fused fissure and chronic obstructive pulmonary disease: a pilot trial comparing sealant and standard treatment. Interact Cardiovasc Thorac Surg 2009;9:973-7. [Crossref] [PubMed]
- Allen MS, Wood DE, Hawkinson RW, et al. Prospective randomized study evaluating a biodegradable polymeric sealant for sealing intraoperative air leaks that occur during pulmonary resection. Ann Thorac Surg 2004;77:1792-801. [Crossref] [PubMed]
- Park BJ, Snider JM, Bates NR, et al. Prospective evaluation of biodegradable polymeric sealant for intraoperative air leaks. J Cardiothorac Surg 2016;11:168. [Crossref] [PubMed]
- Ueda K, Tanaka T, Jinbo M, et al. Sutureless pneumostasis using polyglycolic acid mesh as artificial pleura during video-assisted major pulmonary resection. Ann Thorac Surg 2007;84:1858-61. [Crossref] [PubMed]
- Ueda K, Tanaka T, Li TS, et al. Sutureless pneumostasis using bioabsorbable mesh and glue during major lung resection for cancer: who are the best candidates? J Thorac Cardiovasc Surg 2010;139:600-5. [Crossref] [PubMed]
- Serra-Mitjans M, Belda-Sanchis J, Rami-Porta R. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2005.Cd003051. [PubMed]
- Rice TW, Blackstone EH. Use of sealants and buttressing material in pulmonary surgery: an evidence-based approach. Thorac Surg Clin 2010;20:377-89. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Agzarian J, Fahim C, Shargall Y, et al. The Use of Robotic-Assisted Thoracic Surgery for Lung Resection: A Comprehensive Systematic Review. Semin Thorac Cardiovasc Surg 2016;28:182-92. [Crossref] [PubMed]
- Rajaram R, Mohanty S, Bentrem DJ, et al. Nationwide Assessment of Robotic Lobectomy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;103:1092-100. [Crossref] [PubMed]
- Belda-Sanchis J, Serra-Mitjans M, Iglesias Sentis M, et al. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2010.Cd003051. [PubMed]
- Burt BM, Shrager JB. The Prevention and Management of Air Leaks Following Pulmonary Resection. Thorac Surg Clin 2015;25:411-9. [Crossref] [PubMed]
- Macchiarini P, Wain J, Almy S, et al. Experimental and clinical evaluation of a new synthetic, absorbable sealant to reduce air leaks in thoracic operations. J Thorac Cardiovasc Surg 1999;117:751-8. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 95-6. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Rocco G, Rendina EA, Venuta F, et al. The use of sealants in modern thoracic surgery: a survey. Interact Cardiovasc Thorac Surg 2009;9:1-3. [Crossref] [PubMed]
- Clayton JD, Elicker BM, Ordovas KG, et al. Nonclotted Blood Patch Technique Reduces Pneumothorax and Chest Tube Placement Rates After Percutaneous Lung Biopsies. J Thorac Imaging 2016;31:243-6. [Crossref] [PubMed]


