
Single-center experience of extracorporeal membrane oxygenation mainly anticoagulated with nafamostat mesilate
Introduction
Extracorporeal membrane oxygenation (ECMO) can be a life-saving tool in acute cardiopulmonary collapse. However, ECMO itself is a major cause of life-threatening complications such as thromboembolism because of the artificial surface of the circuit persistently reacts with blood components, especially coagulation factors. To minimize thromboembolic complications and maintain patency of the circuit, proper anticoagulation is essential during ECMO. Unfractionated heparin (UFH) is the most widely used anticoagulant in ECMO owing to its cost-effectiveness and easy reversibility with protamine sulfate. However, bleeding remains a major concern with UFH and can occur in up to 36% of patients (1). Although there is a report about low heparin dose protocol which reduced bleeding complications, the data was not enough to draw the correlation (2). Accordingly, the need for alternative drugs to UFH has been suggested.
Nafamostat mesilate (NM) is a serine protease inhibitor and could inhibit proteinase-activated receptors, resulting in a reduced complement cascade activation, leukocyte activation, and platelet aggregation. Because of its antithrombin and antiplasmin effects, NM has been used clinically in disseminated intravascular coagulopathy and as an anticoagulant in patients on hemodialysis. It has previously been widely accepted as an anticoagulant in hemodialysis due to reduced bleeding complications and its equivocal anticoagulation effect (3). Recently, several studies proposed NM as an alternative anticoagulant to heparin during ECMO because it has fewer bleeding complications and a comparable rate of thromboembolic episodes (4). However, there is also a report that NM increased bleeding complications (5) and this drug still has a debated effect. The aim of this study was to evaluate the clinical outcomes of ECMO on NM with a focus on thromboembolic episodes and bleeding.
Methods
Study population
We retrospectively reviewed 102 consecutive ECMO runs at Chungnam National University Hospital between January 2011 and December 2017. Any ECMO runs that were not anticoagulated for any reason, or had a duration of less than 24 h (n=11), were excluded. Finally, a total of 91 ECMO runs on 87 patients were included in the study.
Endpoints
The primary endpoints were bleeding and thromboembolic episodes. Bleeding episodes included: (I) any bleeding from the cannulation or surgical site requiring surgical intervention; (II) cerebral hemorrhage; (III) gastrointestinal bleeding, and (IV) airway bleeding requiring the initial anticoagulant to be stopped or changed. Thromboembolic episodes included: (I) any intracardiac thrombus or circuit thrombosis; (II) embolic stroke, and (III) any type of image-proven systemic thrombosis.
Anticoagulation management during ECMO
An initial UFH bolus of 50–100 units/kg, according to the patient’s body weight was administered at the time of cannulation for ECMO, typically followed by continuous infusion of NM or UFH. NM was infused hourly at a dose of 0.5 mg/kg of body weight and UFH was infused continuously at a dose of 7.5–20.0 units/kg/h. The therapeutic dose of each anticoagulant was monitored according to the activated coagulation time (ACT) or activated partial thromboplastin time (aPTT). All patients were monitored by at least one of the both methods or both. Target values for ACT were 150 to 200 s, and those for aPTT were 55 to 70 s.
Statistical analysis
Statistical analysis was performed using the IBM SPSS software (ver. 21.0; IBM Corp., Armonk, NY, USA). Categorical variables are expressed as frequencies and percentages. Continuous variables are presented as means ± standard deviation. The chi-square test or Fisher’s exact test was used to compare categorical variables, and Student’s t-test or the Mann-Whitney U test was used to compare continuous variables. Multiple logistic regression models were used to analyze risk factors for bleeding during ECMO. A backward stepwise selective method was used to select the final model. P values <0.05 were considered to indicate statistical significance.
Ethics statement
The present study was reviewed and approved by the ethics committee of Chungnam National University. The requirement for individual consent from patients or relatives was waived accordingly.
Results
Descriptive data analysis
During the study period, a total of 91 ECMO runs on 87 patients were identified. Of the 87 patients, 47 (54.0%) patients were successfully weaned and 29 (33.3%) survived to discharge. Most of the runs were anticoagulated with NM (n=68, 74.7%), followed by heparin (n=22, 24.2%) and argatroban (n=1, 1.1%). The mean duration of ECMO support was 11.3±11.1 days. The overall incidence of bleeding was 46.2% (n=42) and that of thromboembolic episodes was 12.1% (n=11). In the NM group, the incidence of hyperkalemia requiring any type of intervention was 17.6% (n=12). Many of the baseline characteristics between the UFH group and NM group were not significantly different (Table 1). However, the UFH group had significantly more venovenous (VV) ECMO and respiratory cases. On the other hand, this group had fewer cases of post-cardiotomy syndrome.
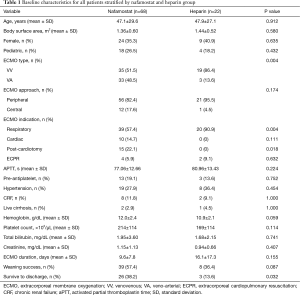
Full table
NM vs. UFH
The mean level of aPTT during ECMO run was not significantly different between two groups (NM: 77.06±12.66 s vs. UFH: 80.96±13.43 s, P=0.224). Regarding the primary endpoints, the NM group tended to experience less bleeding than the UFH group (38.2% vs. 72.7%, P=0.005). However, there were 3 cases of cerebral hemorrhage in the NM group on contrary to the UFH group which was none. There were no significant differences in terms of thromboembolic episode rates (13.2% vs. 9.1%, P=1.000) (Table 2). However, regarding major thromboembolic complications, there were 3 case of cerebral infarct in the NM group which was none in the UFH group.
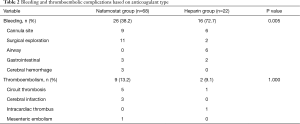
Full table
Predictors of bleeding
Tables 3,4 show the results of univariate and multivariate analyses, respectively, regarding bleeding risk factors during ECMO. In the univariate analysis, heparin use [hazard ratio (HR), 4.308; 95% confidence interval (CI), 1.495–12.410] and low platelet count (HR, 3.855; 95% CI, 1.241–11.980) were significantly associated with bleeding complications during ECMO. However, in the multivariate analysis, the use of heparin (HR, 4.372; 95% CI, 1.449–13.190) was the only independent predictor of bleeding complications.
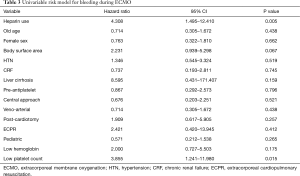
Full table

Full table
Discussion
Currently, ECMO is one of the most important life-sparing methods in patients with acute cardiopulmonary failure (6). However, contrary to its ease of accessibility and efficiency of resuscitation, ECMO carries a potential risk of thrombosis (7). Continuous contact between circulating blood and the foreign surface of the ECMO circuit shifts the normal physiologic hemostatic balance to a hypercoagulable state. To suppress this shift, the use of anticoagulation during ECMO is essential. UFH is the most widely used anticoagulant in ECMO owing to its cost-effectiveness and easy reversibility with protamine sulfate. However, there remains the problem of bleeding complications related to systemic heparinization; this occurs in up to 60% of patients and directly affects prognosis (8). This concern lead to low heparin dose protocol to reduce bleeding complications during ECMO but the correlation is not clear (2).
NM is a serine protease inhibitor that attenuates coagulation, fibrinolysis, and platelet aggregation. Previously, NM was widely accepted as an anticoagulant in hemodialysis because of reduced bleeding complications and its equivocal anticoagulation effect (3). Recently, several studies proposed NM as an alternative anticoagulant to heparin during ECMO. In their large animal experimental study, Han et al. reported that NM showed a similar anticoagulation effect to UFH according to thromboelastography results. Additionally, they noted that NM had an anti-inflammatory effect during ECMO (9). Moreover, the heparin group (60.8%) had more complications related to bleeding than the NM group (23.5%). They also reported that the NM group received significantly fewer transfusions (4). Lim et al., however, reported conflicting results in their clinical study, they found that bleeding complications were significantly higher in the NM group in both the unmatched and matched cohorts (P=0.03), whereas thromboembolic events were comparable (5). The reason for their conflicting results might be related to different baseline characteristics between two groups. In their study, NM group tended to have more liver cirrhosis and low platelet count which might contributed to increased incidence of bleeding complications. Other contribution factors might be their less mean duration of ECMO time (<100 h) and selected indication for ECMO [100% veno-arterial (VA) ECMO cases].
In the present study, the overall incidence of bleeding was 46.2%, which is higher than that of other reported studies. This result might be due to the use of a much wider definition of bleeding complications. However, we followed the definition of the Extracorporeal Life Support Organization (10). The main source of bleeding was the cannulation site, followed by the surgical site, airway, gastrointestinal system, and brain. This is similar to a previous study by Aubron et al. (8) which compared NM and UFH groups. In that study, bleeding was significantly higher in the UFH group (72.7%). Even though the UFH group had more cases of VV and fewer of post-cardiotomy indication in our study, this would not contribute to the difference between the studies, because the VV type is usually a protective factor and post-cardiotomy indication is a risk factor for bleeding complications.
Although NM group had less cases of bleeding complications in our study, the concern is rate of major complications regarding cerebral hemorrhage and cerebral infarction was much higher in NM group. It might be related to preexisting risk factors for cerebrovascular accident in NM group. Because the NM group had more cardiac cases, especially post-cardiotomy cases on contrary to UFH group which had more respiratory cases.
From the viewpoint of cost, absolute cost of NM is about 5 times higher than UFH (1 ample of 50 mg NM ≒ $10 USD vs. 1 ample of 5,000 unit UFH ≒ $2 USD). This cost difference can be the one of limitations of NM because usual continuous infusion dosage of NM is 10–15 mg/h and UFH is 500–1,000 units/h during ECMO.
There are several known predictors of bleeding during ECMO. Previously, Kasirajan et al. reported that heparin use and thrombocytopenia have a positive correlation with intracranial hemorrhage during ECMO (11). Werho et al. reported that post-cardiotomy indication is an independent risk factor for hemorrhagic complications during ECMO, especially in pediatric patients (12). Smith et al. showed that cardiac and extracorporeal cardiopulmonary resuscitation patients tend to receive significantly more red blood cell transfusions during ECMO (13). In our analysis, the use of heparin and a low platelet count predicted bleeding on univariate analysis. Finally, according to multivariate analysis, heparin use was the major bleeding risk factor during ECMO. However, other reported risk factors, such as low fibrinogen level (14) and preoperative coagulation abnormalities (15) were not considered in our analysis; these unmeasured confounders may have affected the results.
Several limitations of our study should be noted. First, it used a retrospective, single-institution design and the number of subjects in the UFH group was relatively small. Second, we are not certain that the anticoagulation in the NM group was completely effective because there is no consensus regarding the most effective NM regimen during ECMO. Third, our study only focused on the incidence and predictors of bleeding complications; the impact of bleeding on clinical outcome, such as mortality, was not considered. Lastly, unmeasured factors may have acted as confounders.
In conclusion, NM appears to be associated with fewer bleeding complications during ECMO and does not increase the incidence of thromboembolic episodes, although it should be borne in mind that this was a small study. However, further studies with larger numbers of cases and a prospective design should be performed to validate our findings.
Acknowledgments
None.
Footnote
Conflicts of Interest: Meeting presentation: The 50th Anniversary of the Korean Society for Thoracic and Cardiovascular Surgery in conjunction with the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG2018).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was reviewed and approved by the ethics committee of Chungnam National University (No. CNU 2019-05-049). The requirement for individual consent from patients or relatives was waived accordingly.
References
- Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc 2013;15:172-8. [PubMed]
- Martucci G, Panarello G, Occhipinti G, et al. Anticoagulation and Transfusions Management in Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome: Assessment of Factors Associated With Transfusion Requirements and Mortality. J Intensive Care Med 2019;34:630-9. [PubMed]
- Ohtake Y, Hirasawa H, Sugai T, et al. Nafamostat mesylate as anticoagulant in continuous hemofiltration and continuous hemodiafiltration. Contrib Nephrol 1991;93:215-7. [Crossref] [PubMed]
- Han SJ, Kim HS, Kim KI, et al. Use of Nafamostat Mesilate as an Anticoagulant during Extracorporeal Membrane Oxygenation. Journal of Korean Medical Science 2011;26:945. [Crossref] [PubMed]
- Lim JY, Kim JB, Choo SJ, et al. Anticoagulation During Extracorporeal Membrane Oxygenation; Nafamostat Mesilate Versus Heparin. Ann Thorac Surg 2016;102:534-9. [Crossref] [PubMed]
- Abrams D, Combes A, Brodie D. Extracorporeal Membrane Oxygenation in Cardiopulmonary Disease in Adults. Journal of the American College of Cardiology 2014;63:2769-78. [Crossref] [PubMed]
- Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. Asaio j 2017;63:60-7. [Crossref] [PubMed]
- Aubron C, DePuydt J, Belon F, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Annals of Intensive Care 2016;6:97. [PubMed]
- Han SJ, Han W, Song H-J, et al. Validation of Nafamostat Mesilate as an Anticoagulant in Extracorporeal Membrane Oxygenation: A Large-Animal Experiment. The Korean Journal of Thoracic and Cardiovascular Surgery 2018;51:114-21. [Crossref] [PubMed]
- . . Available online: https://www.elso.org/Portals/0/Files/elsoanticoagulationguideline8-2014-table-contents.pdfAnticoagulation Guideline ELSO. 2014.
- Kasirajan V, Smedira NG, McCarthy JF, et al. Risk factors for intracranial hemorrhage in adults on extracorporeal membrane oxygenation. Eur J Cardiothorac Surg 1999;15:508-14. [Crossref] [PubMed]
- Werho DK, Pasquali SK, Yu S, et al. Hemorrhagic complications in pediatric cardiac patients on extracorporeal membrane oxygenation: an analysis of the Extracorporeal Life Support Organization Registry. Pediatr Crit Care Med 2015;16:276-88. [Crossref] [PubMed]
- Smith A, Hardison D, Bridges B, et al. Red blood cell transfusion volume and mortality among patients receiving extracorporeal membrane oxygenation. Perfusion 2013;28:54-60. [Crossref] [PubMed]
- Doymaz S, Zinger M, Sweberg T. Risk factors associated with intracranial hemorrhage in neonates with persistent pulmonary hypertension on ECMO. J Intensive Care 2015;3:6. [Crossref] [PubMed]
- Kalbhenn J, Wittau N, Schmutz A, et al. Identification of acquired coagulation disorders and effects of target-controlled coagulation factor substitution on the incidence and severity of spontaneous intracranial bleeding during veno-venous ECMO therapy. Perfusion 2015;30:675-82. [Crossref] [PubMed]


