
Searching for an arrow hitting two targets: the time to evaluate long-term outcomes of video-assisted thoracoscopic surgery lobectomy for early-stage lung cancer
Lung cancer is the leading cause of cancer deaths worldwide. Surgery has been a key element of definitive therapy for lung cancer since the first successful en-bloc left-sided pneumonectomy by Graham in 1933 (1). The evolution of lung cancer surgery has been driven by thoracic surgeons’ enthusiasm to achieve maximal oncological outcomes with minimal invasiveness; these two factors are essential in establishing the optimal surgical approach for lung cancer. In the 1950s and 1960s, lobectomy eventually replaced pneumonectomy for its safety and curative efficacy (2). The landmark Lung Cancer Study Group-821 study, the only completed randomized trial to date comparing lobectomy with sublobar resection, strongly supported thoracotomy lobectomy over thoracotomy sublobar resection for better oncological outcomes (3). For the past 60 years, lobectomy via thoracotomy with hilar and mediastinal lymph node dissection or sampling has been the standard of care for early stage (I or II) lung cancer.
Video-assisted thoracoscopic surgery (VATS) lobectomy has been increasingly utilized worldwide for early stage lung cancer since its introduction in the 1990s (4), accounting for 20% to 60% of all lobectomy cases in the 2010s (5,6). Accumulating evidence has shown that the VATS approach is associated with improved short-term outcomes: lesser pain, fewer postoperative complications, shorter length of hospital stay, and similar or lower perioperative mortality compared to the thoracotomy approach (7-9). However, it remains unclear whether long-term oncological outcomes of VATS are better than or equivalent to those of open thoracotomy. Two systematic reviews in the 2000s indicated the advantages of VATS over thoracotomy (10,11); however, incomplete nodal dissection is a possible disadvantage of VATS lobectomy (7). Therefore, the current National Comprehensive Cancer Network guideline defines VATS lobectomy as “an acceptable approach for patients with surgically resectable tumors as long as the principles of thoracic surgery are not compromised” (12).
There have been several reports using propensity score-matching to compare long-term outcomes of VATS lobectomy to those of thoracotomy lobectomy (Table 1) (13-18). Among these, Yang et al. in their National Cancer Database-based retrospective study reported that VATS did not compromise the oncologic outcomes of early-stage non-small cell lung cancer (NSCLC) (18). This study analyzed 1,464 propensity score-matched pairs of patients with cT1-2N0M0 (as per 7th TNM classification) undergoing VATS or open thoracotomy lobectomy. Propensity scores, defined as the probability of treatment with VATS approach versus thoracotomy, were determined considering age, sex, race, Charlson/Deyo comorbidity score, education and income levels, insurance type, distance from facility, facility type, T-status, tumor size, tumor location, histology, and grade to reduce bias. VATS approach was associated with a shorter length of hospital stay (VATS median 5 days vs. thoracotomy 6 days; P<0.001) and an equivalent 30-day mortality (VATS 1.7% vs. thoracotomy 2.3%; P=0.50) compared to the thoracotomy approach. Further, the number of lymph nodes harvested with VATS was higher than with thoracotomy, but the difference was not statistically significant {VATS median 9 [interquartile range (IQR), 5–16] vs. thoracotomy median 9 [IQR, 5–14]; P=0.053}. Moreover, there was no statistically significant difference in the rates of nodal upstaging to either pN1 (VATS 7.1% vs. thoracotomy 8.4%; P=0.18) or pN2 (VATS 4.5% vs. thoracotomy 3.9%; P=0.45) between the two approaches. Importantly, the 5-year overall survival of the VATS group was equivalent to that of the thoracotomy group (VATS 66.3% vs. thoracotomy 65.8%; P=0.92). The subgroup analyses on patients with no comorbidities and on those who had more than 11 lymph nodes evaluated showed similar results. These results support that VATS lobectomy is associated with a less invasive process and equivalent oncological outcome for early-stage NSCLC compared to thoracotomy lobectomy.
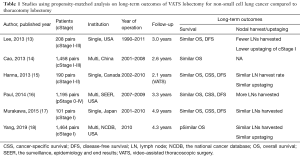
Full table
The study by Yang et al. (18) has the following advantages: (I) the study population was largest among all the studies conducted to date, (II) the year of surgery was limited to 2010, (III) the duration of postoperative follow-up seemed acceptable for evaluation of 5-year survival rates (median 4.3 years), and (IV) propensity-score matching was utilized to minimize bias. It is important to limit the year of study period (i.e., the year of surgery) in order to consider the changes in the techniques and technologies concerning lung cancer surgery over time. There were also some disadvantages of the study, as follows: (I) it followed a retrospective design, (II) cancer-specific or recurrence-free survival rates were not evaluated, (III) no clear definitions of “VATS” or “thoracotomy” were described, and (IV) detailed information on preoperative comorbidities, intraoperative findings (such as duration of operation and blood loss), and postoperative complications was not included.
A major factor that complicates studies comparing VATS lobectomy with thoracotomy lobectomy is the heterogeneity in surgeons’ skill, surgical procedures and patients’ background (i.e., ethnicity, frailty, and comorbidity). This heterogeneity might be reflected by the difference in 30-day mortality of lobectomy among the Society of Thoracic Surgeons, European Society of Thoracic Surgeons, and Japanese Association for Thoracic Surgery (1.4%, 2.6%, and 0.3%, respectively) (5,6).
Since the term VATS comprises various and heterogenous sets of procedures, the international VATS consensus statement in 2014 defined the following criteria (19): (I) non-rib-spreading; (II) a maximum length of 8 cm for utility incision; (III) individual dissection of pulmonary vessels and bronchus; and (IV) standard node sampling or dissection. Considering the rapid advances in VATS, including the development of uniportal or non-intubated techniques, the definition of VATS will vary. Clinicians need to understand which VATS approach is utilized for their patients, based on up-to-date knowledge on progress in lung cancer surgery.
A recent study showed an increasing number of patients with a high risk for open lobectomy undergoing VATS lobectomy (20). This indicates that VATS is better tolerated than standard thoracotomy by patients with poor organ function. However, there are a few limitations of VATS, such as a poor cardiopulmonary reserve intolerable to one-lung ventilation.
Undoubtedly, lung cancer itself is a heterogenous entity. Specifically, pulmonary adenocarcinoma has a wide histological spectrum, ranging from adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA) to more aggressive subtypes such as micropapillary predominant adenocarcinoma. The Japan Clinical Oncology Group (JCOG) 0804 study showed sublobar resection to achieve a 5-year recurrence-free survival of 99.7% for peripheral and small (total size ≤2 cm) cTis/T1mi ground-glass nodule (GGN), the radiological correlate of AIS, MIA or lepidic adenocarcinoma (21,22). However, when the tumor showed ≥5% of micropapillary component or presence of spread through air spaces, sublobar resection of cT1a/T1b disease became suboptimal due to the increased risk of recurrence (23,24). The JCOG 0802 study, a randomized controlled trial comparing lobectomy with segmentectomy for cT1a/T1b pulmonary nodule, will hopefully provide more information in the upcoming years. It is indicated that radiological parameters such as the ratio of solid size to total size in part-solid GGN and histological features are promising biomarkers to determine the extent of pulmonary resection.
In the past two decades, there have been enormous advancements in systemic therapy of NSCLC, such as tyrosine kinase inhibitors and immune-checkpoint inhibitors. Further research will lead to a better understanding of lung cancer biology and ultimately help in optimizing personalized targeted therapy. According to the International Association for the Study of Lung Cancer database, the proportion of patients with lung cancer undergoing surgery alone or as part of a multimodal treatment increased from 54% in the 1990s to 85% in the 2000s (25). While this observation might not directly reflect a “real-world” scenario, it suggests the increased possibility of lung cancer patients receiving suitable surgical treatments in various settings.
In the era of precision medicine, it is essential to identify the optimal surgical approach in case-specific scenario, based on the patients’ frailty or comorbidity, lung cancer biology, and the timing of surgery. In the future, omics research, identification of suitable biomarkers, and artificial intelligence will aid in accurate preoperative risk assessment and selection of the best surgical approach for lung cancer. Advancements in lung cancer surgery continue to identify the optimal surgical approach that is suitable for patients with various conditions and for eliminating different types of cancer cells—an arrow hitting two targets.
Acknowledgments
We would like to thank Editage (HYPERLINK “http://www.editage.jp” www.editage.jp) for English language editing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Graham EA, Singer JJ. Successful removal of an entire lung for carcinoma of the bronchus. JAMA 1933;101:1371-4. [Crossref] [PubMed]
- Churchill ED, Sweet RH, Soutter L, et al. The surgical management of carcinoma of the lung; a study of the cases treated at the Massachusetts General Hospital from 1930 to 1950. J Thorac Surg 1950;20:349-65. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg 1992;104:1679-85; discussion 1685-7.
- Seder CW, Salati M, Kozower BD, et al. Variation in pulmonary resection practices between the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons General Thoracic Surgery Databases. Ann Thorac Surg 2016;101:2077-84. [Crossref] [PubMed]
- Shimizu H, Endo S, Natsugoe S, et al. Thoracic and cardiovascular surgery in Japan in 2016: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2019;67:377-411. [Crossref] [PubMed]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal upstaging is more common with thoracotomy than with VATS during lobectomy for early-stage lung cancer: An analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014;148:637-43. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-18.
- National Comprehensive Cancer Network. Non-small cell lung cancer (Version 4. 2019).
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2013;96:951-60; discussion 960-1. [Crossref] [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [Crossref] [PubMed]
- Hanna WC, de Valence M, Atenafu EG, et al. Is video-assisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy? Eur J Cardiothorac Surg 2013;43:1121-5. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575. [Crossref] [PubMed]
- Murakawa T, Ichinose J, Hino H, et al. Long-term outcomes of open and video-assisted thoracoscopic lung lobectomy for the treatment of early stage non-small cell lung cancer are similar: a propensity-matched study. World J Surg 2015;39:1084-91. [Crossref] [PubMed]
- Yang CJ, Kumar A, Klapper JA, et al. A National analysis of long-term survival following thoracoscopic versus open lobectomy for stage I non-small-cell lung cancer. Ann Surg 2019;269:163-71. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Donahoe LL, de Valence M, Atenafu EG, et al. High risk for thoracotomy but not thoracoscopic lobectomy. Ann Thorac Surg 2017;103:1730-5. [Crossref] [PubMed]
- Suzuki K, Watanabe S, Wakabayashi M, et al. A nonrandomized confirmatory phase III study of sublobar surgical resection for peripheral ground glass opacity dominant lung cancer defined with thoracic thin-section computed tomography (JCOG0804/WJOG4507L). J Clin Oncol 2017;35:8561. [Crossref]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204-23.
- Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2 cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24.


