Electromagnetic navigation bronchoscopy fluorescence localization and VATS subxiphoid bilateral wedge resection under non-intubated anesthesia
Introduction
Computed tomography guided (CT guided) methylene blue or medical glue injection is the prevailing methods for bilateral pulmonary sub-solid nodules (SSN). Most of the bilateral SSNs are treated by surgeries on either side in different period. As the electromagnetic navigation system developed, reports showed reliable results of minimal invasiveness and precision in pulmonary nodule localization (1). Non-intubated anesthesia in pulmonary surgeries has been reported as a feasible and safe method with shorter anesthesia and recovery time (2). Besides, the feasibility and convenience of subxiphoid video-assisted thoracoscopic surgery (VATS) has been reported and it is considered as less painful and minimally invasive thoracic surgery approach (3,4). To validate precision and minimal invasion of these surgical and anesthetic techniques, we performed a bilateral SSNs case treated by VATS subxiphoid bilateral wedge resection with near-infrared (NIR) thoracoscopy under non-intubated anesthesia using electromagnetic navigation bronchoscopy (ENB) fluorescence indocyanine green (ICG) localization.
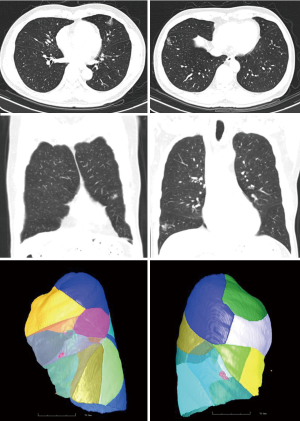
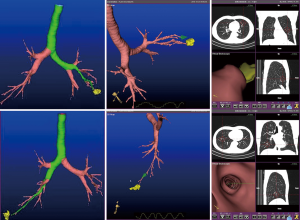
A 62-year-old male asymptomatic patient with body mass index of 21.9 kg/m2 was diagnosed as bilateral pulmonary SSNs by routine body check. The chest CT scan showed a 20 mm × 15 mm and a 20 mm × 10 mm irregular shape of SSNs in right lower lobe anterior base segment and left upper lobe inferior lingular segment with signs of visceral pleural traction (Figure 1). Lung function showed FEV1/FVC was 68%. CT images were collected and imported into the electromagnetic navigation system (LUNGCARE, China) and a virtual bronchus tree was created subsequently. The navigation tracks to the target region were planned and demonstrated on the screen (Figure 2).
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Operative techniques
Total intravenous anesthesia was used to sedate the patient with midazolam (0.05–0.1 mg/kg) and propofol target-control infusion (TCI) mode (1.5–2.0 μg/mL). Sufentanil (10 μg) was administered for analgesia. Anesthesia was maintained with propofol in TCI mode (1.0–3.0 μg/mL), remifentanil (0.03–0.05 μg/kg/min) and dexmedetomidine (0.5–1.0 μg/kg/h). Oxygen (50–60%) was delivered using a laryngeal mask at a flow of 3–3.5 mL/min. Spontaneous respiration was maintained during the whole surgery procedure.
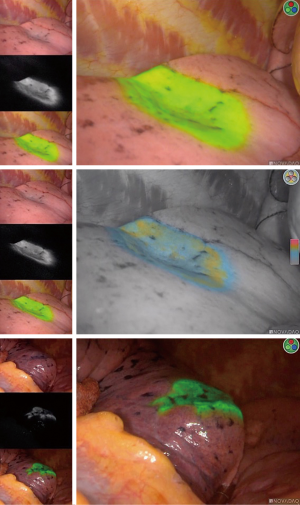
Patient was placed in dorsal decubitus position on the electromagnetic bed. A bronchoscopy was introduced through the laryngeal mask to the airway with the guide wire in the working channel. The ENB was inserted along the navigated track to the targeted segmental bronchiole. A dose of 2 mL ICG (0.25 mg/mL) and 50 mL air was infused through the working channel. The same procedure was performed on the other side of SSN. The patient was moved to the surgical bed after the localization procedure without any sign of ICG reflux.
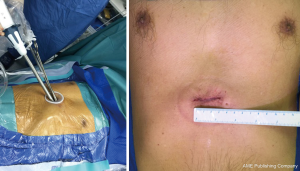
A 3 cm subxiphoid incision was made for both operation and observation (Figure 3). We used harmonic knife to dissect the anterior mediastinal tissue and exposed right lower lobe. Lidocaine (5 mL of 1%) was sprayed on the surface of the lung to prevent coughing. ICG fluorescence was detected by the NIR thoracoscopy and fluorescence imaging system (PINPOINT, Stryker, USA). The pulmonary intersegmental plane was identified clearly (Figure 4). We therefore completed the wedge resection according to the fluorescence intersegmental margin. The same procedure was performed on the left upper lobe. Two SSNs were completely resected 2 cm away from the resected margins grossly and diagnosed as minimal invasive adenocarcinoma in frozen section. We therefore inserted two 14-F chest tubes in each side of chest cavity through the subxiphoid incision for air drainage and removed them when the lungs were fully inflated. The incision was closed with absorbable sutures (Figure S1).

The total operation time including ENB localization and bilateral wedge resections was 80 minutes. It took 40 minutes to finish the bilateral SSNs localizations. Patient was able to eat and drink 4 hours after surgery. Neither pneumothorax nor hemothorax was observed during postoperative follow-up. Patient complained only mild pain on the incision without requiring additional analgesia. The final pathological results were in concordance with frozen section.
Comments
There are advantages to this method. ICG could be hardly noticed in gross examination but only be detected through NIR thoracoscopy. It demonstrates a steady and persistent fluorescence image without compromising pathological examination. It also provides a clear anatomic margin for surgeon to avoid from mis-resection or over-resection. Principally, ENB localization has much lower rate of complications including hemorrhage and pneumothorax than CT-guided puncture (5). Additionally, our previous studies showed non-intubated anesthesia favored shorter recovery time without additional complication (6). Referring to the subxiphoid approach, it provides a method to resect bilateral SSNs in dorsal position rather than two different lateral decubitus positions, which can reduce anxiety, discomfort and financial cost of the patients (7).
In summary, we reported a case of ENB fluorescence localization and VATS subxiphoid bilateral wedge resections under non-intubated anesthesia. We have concluded that ENB ICG injection provides a clear and well-visualized intersegmental margin for resection without interfering with the frozen section examination or increasing complications. This method in combination with NIR thoracoscopy subxiphoid approach under non-intubated anesthesia favors minimal invasiveness and rapid recovery without compromising safety. The optimized patients with bilateral peripheral SSNs are potential candidates for this technique.
Acknowledgments
Funding: This study was supported by National Key R&D Program of China (2017YFC0112700).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hachey KJ, Digesu CS, Armstrong KW, et al. A novel technique for tumor localization and targeted lymphatic mapping in early-stage lung cancer. J Thorac Cardiovasc Surg 2017;154:1110-8. [Crossref] [PubMed]
- Cui F, Liu J, Li S, et al. Tubeless video-assisted thoracoscopic surgery (VATS) under non-intubated, intravenous anesthesia with spontaneous ventilation and no placement of chest tube postoperatively. J Thorac Dis 2016;8:2226-32. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Suda T, Ashikari S, Tochii S, et al. Single-incision subxiphoid approach for bilateral metastasectomy. Ann Thorac Surg 2014;97:718-9. [Crossref] [PubMed]
- Anayama T, Hirohashi K, Miyazaki R, et al. Near-infrared dye marking for thoracoscopic resection of small-sized pulmonary nodules: comparison of percutaneous and bronchoscopic injection techniques. J Cardiothorac Surg 2018;13:5. [Crossref] [PubMed]
- Li S, Jiang L, Ang KL, et al. New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg 2017;51:689-93. [PubMed]
- Chiu CH, Chao YK, Liu YH. Subxiphoid approach for video-assisted thoracoscopic surgery: an update. J Thorac Dis 2018;10:S1662-5. [Crossref] [PubMed]
- He J, Yang H, He J, et al. The process of electromagnetic bronchoscopy navigation localization and subxiphoid uniportal VATS bilateral wedge resections. Asvide 2019;6:208. Available online: http://www.asvide.com/watch/32892