
Diagnostic value of CD206+CD14+ macrophages in diagnosis of lung cancer originated malignant pleural effusion
Introduction
Pleural effusions are common clinical complications, with the accumulation of fluid in the pleural space. They can be caused by various diseases, including cancer, inflammation and viral infection (1). Malignant pleural effusion (MPE) occurs in 50% of metastatic malignancy and indicates poor prognosis and short life expectancy (2,3). Differentiating MPE from benign pleural effusion remains a severe challenge. Microbiological and cytological examinations are routine methods to diagnose MPE, however, their diagnostic values vary from 40% to 80% (4). Therefore, novel and efficient biomarkers are required for the diagnosis of MPE.
The cellular environment is complex in MPE, including immune cells, lymphocytes and bone marrow-derived inflammatory cells. As a pivotal component of host immune cells, macrophages play a vital role in immune response. According to different physiological conditions, macrophages can be polarized into a classically activated (M1) phenotype or an alternatively activated (M2) phenotype. M1 macrophages express CD86, CD80, HLA-DR and promote T helper type 1 (Th1) response by secreting pro-inflammatory cytokines, whereas M2 macrophages promote T helper type 2 (Th2) response by secreting anti-inflammatory cytokines and support tumor development by promoting tumor growth, metastasis, and angiogenesis (5,6). Macrophages existing in tumor microenvironment are always known as tumor-associated macrophages, which are similar to M2 phenotype.
The macrophage mannose receptor CD206 is commonly used as a pan marker of M2 macrophages both in vivo and in vitro. Previous studies have demonstrated that CD206+ macrophages could function as a hallmark in several types of cancer. Dong and his colleagues reported that CD206+ macrophages was a good prognostic indicator for hepatocellular carcinoma (7). However, the significance of CD206+ macrophages in MPE has not been described yet. In this study, we analyzed the phenotypes of macrophages in both MPE and benign pleural effusion. CD14 was used as a pan marker for macrophages, while CD86 and CD206 were used as markers for M1 and M2, respectively. We aim to figure out whether CD206+CD14+ macrophages could be used as a reliable marker for diagnosing MPE.
Methods
Study populations
The study protocol was approved by the ethics committee of Beijing Chao-Yang Hospital. From January 2018 to December 2018, a total of 60 patients with definite diagnosis of pleural effusion were enrolled in our study, and they all signed the informed consent according to the approved guideline.
For the MPE group, MPE was obtained from 34 patients (24 male, 10 female, and aged between 38 to 87 years) with lung cancer. The diagnosis of MPE was established by the appearance of cancer cells in pleural effusion and/or on closed pleural biopsy samples. Hematoxylin and eosin (H&E) staining of pleural effusions was performed, and the results were evaluated by microscopy to confirm MPE (Figure S1). Histologically, 21 subjects were adenocarcinoma, 4 were squamous cell carcinoma, 2 were small cell lung cancer, and 7 were undetermined lung cancer.
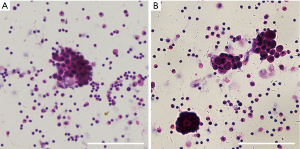
Twenty-six patients (16 male, 10 female, aged between 21 and 85 years) with pleural effusion caused by tuberculosis (n=17), pneumonia (n=6), or heart failure (n=3) were defined as the benign pleural effusion group. Patients were not recruited in our study if they were subjected to thoracic injury or if there were any invasive operations performed into the thoracic cavity within 3 months prior to their hospitalization. All patients had no history of prior treatment for tuberculosis or cancer. The subjects included in the study were all HIV-negative, with no history of corticosteroids usage or any other drugs known to affect the immunological condition.
Sample collection and processing
Pleural fluid sample (100–150 mL) from each subject was collected in tubes containing heparin when the diagnostic thoracentesis was performed within 24 hours after hospitalization. Peripheral blood sample (4 mL) was drawn at the same time. Pleural effusion and peripheral blood samples were immersed into ice and then centrifuged at 400 g for 6 minutes at 4 °C. After centrifugation, the supernatants were stored at −80 °C and the cell pellets were then resuspended in phosphate buffer saline (PBS). Mononuclear cells were isolated using Lymphocyte Separation Medium (MP Biomedicals) according to the manufacturer’s instructions.
Flow cytometry analysis
Red blood cells were lysed, and the isolated mononuclear cells were incubated with fluorochrome-conjugated antibodies specific for human for 20 min in the dark. These antibodies, including anti-CD45, anti-CD14, anti-CD206, and anti-CD86 mAbs, were purchased from Thermo Fisher Scientific (Waltham, MA, USA) or BD Biosciences (San Diego, CA, USA). Cells were then fixed in 4% paraformaldehyde. All experiments were analyzed by flow cytometry on a FACS Canto II (BD Biosciences). The analyses were performed with FCS Express 5 software (De Novo Software, Los Angeles, CA, USA).
Statistical analysis
Data were expressed as mean ± SEM. The significance of differences of data between the two groups was analyzed by using Student’s t test. Receiver operating characteristic (ROC) analysis were analyzed, and areas under the ROC curve (AUC) were calculated to evaluate the diagnostic value of CD14+, CD86+CD14+ and CD206+CD14+ macrophages to discriminate between MPE and benign pleural effusion. Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio and negative likelihood ratio were also analyzed (8). These parameters and their 95% confidence intervals (CIs) were also evaluated together in this study. The optimal cutoff points were established on their maximum Youden’s index. The Statistical Program for Social Science (SPSS) 19.0 and MedCalc software were used to conduct the analyses, and differences were assumed significant when P<0.05.
Results
Clinical and demographic parameters of patients with pleural effusions
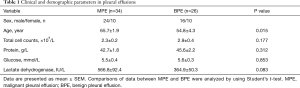
Some clinic pathological characteristics of patients with MPE and benign pleural effusion are summarized in Table 1. In this study, patients with MPE were elder than those with benign pleural effusion (P=0.015). There is no difference in the cytological, clinical and demographic characteristics of pleural effusions between the two groups (all P>0.05).
Full table
Numbers of CD14+, CD86+CD14+ and CD206+CD14+ macrophages in pleural effusions
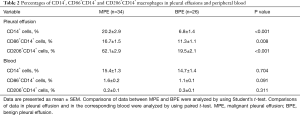
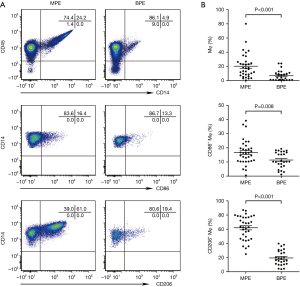
As shown in Table 2 and Figure 1, the percentages of CD14+, CD86+CD14+ and CD206+CD14+ macrophages in MPE were statistically higher than those in benign pleural effusion (95% CIs for the differences were 6.2% to 20.5%, 1.4% to 9.3% and 35.0% to 50.1%, respectively; all P<0.05). In addition, CD86 and CD206 were barely detected in the peripheral blood monocytes. The numbers of CD86+CD14+ and CD206+CD14+ cells in MPE were statistically higher than their corresponding ones in blood (95% CIs for the differences were 12.0% to 18.1% and 56.1% to 67.6%, respectively; both P<0.001). The percentages of CD86+CD14+ and CD206+CD14+ cells in benign pleural effusion were also much higher than those in blood (95% CIs for the differences were 7.9% to 12.4% and 15.0% to 23.4%, respectively; both P<0.001).
Full table
Diagnostic values of CD14+, CD86+CD14+, and CD206+CD14+ macrophages in pleural effusions
The high levels of CD14+, CD86+CD14+ and CD206+CD14+ macrophages in MPE indicated that they may serve as potential biomarkers to diagnose MPE. Thus, their diagnostic values to discriminate malignant from benign pleural effusion were analyzed with the ROC analyses. The AUC of CD14+ macrophages in the diagnosis of MPE was 0.788 at a cutoff level of 10.9% (sensitivity, 64.7%; specificity, 80.8%). The AUC of CD86+CD14+ macrophages to diagnose MPE was 0.698 with a cutoff level of 10.2% (sensitivity, 88.2%; specificity, 50.0%). The highest diagnostic accuracy was achieved in CD206+CD14+ macrophages with the AUC of 0.980 at the cutoff level of 39.8% (sensitivity, 88.2%; specificity, 100.0%) (Figure 2).
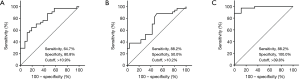
The diagnostic parameters of CD14+, CD86+CD14+ and CD206+CD14+ macrophages in pleural effusions, including sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, positive predictive value and negative predictive value, are presented in Table 3. Based on these parameters, it was distinguished that CD206+CD14+ macrophages had the highest diagnostic performance compared with CD14+ and CD86+CD14+ macrophages.

Full table
Discussion
MPE is a distressing condition which occurs at advanced stages of various malignancies. In the United States, almost 40,000 people are affected by MPE every year, and patients suffering from MPE usually face worse life quality and poor prognosis (2). The diagnosis between malignant and benign pleural effusion remains a difficult problem, since a large variation in diagnostic accuracy of pleural fluid cytology ranges from 60% to 90% (9,10). Tumor markers are not suitable for diagnosing MPE, because their sensitivity and specificity are relatively low (11,12). Medical thoracoscopy is considered to be a gold-standard method in diagnosing MPE. However, as an invasive technique, it may cause complications and increase morbidity (13-15). Therefore, novel tests and markers are called for discriminating between MPE and benign pleural effusion. As far as we know, our current study is the first attempt to evaluate the diagnostic value of CD206+CD14+ macrophages in MPE.
There are plentiful studies demonstrated the clinical significance of CD86+ and CD206+ macrophages in various disease. In colorectal cancer, CD86+ macrophages were used to indicate a better prognosis (16). In hepatocellular carcinoma, CD206+ macrophages in primary tumor sites were associated with poor prognosis (17). In renal cell carcinoma, high percentage of CD206+ macrophages could be used as a promising biomarker for poor survival (18). However, the role of CD86+ and CD206+ macrophages in MPE is poorly understood. Therefore, we present the first data on the percentages of CD14+, CD86+CD14+ and CD206+CD14+ macrophages in malignant and benign pleural effusions and analyze their diagnostic values for the diagnosis of MPE.
Previously, Chen and his colleagues reported that combinations of VEGF mRNA and endostatin mRNA provided a high-diagnostic value (sensitivity, 95.7%; specificity, 88.9%) for diagnosis of MPE (19). Pleural fluid cf-DNA integrity index was predicted as a promising biomarker for MPE with a sensitivity of 92% and a specificity of 92.6% (20). Wang et al. reported that CD163+CD14+ macrophages were indicated as a helpful marker for MPE with a sensitivity of 81.2% and a specificity of 100% (21). Our study showed that the percentages of CD14+, CD86+CD14+ and CD206+CD14+ macrophages in MPE were statistically higher than those in benign pleural effusion. At a cutoff level of 10.9%, the sensitivity and specificity of CD14+ macrophages to diagnose MPE were 64.7% and 80.8%, respectively. At a cutoff value of 10.2%, the sensitivity and specificity of CD86+CD14+ macrophages in discriminating MPE from benign pleural effusion were 88.2% and 50.0%, respectively. At a cutoff level of 39.8%, CD206+CD14+ macrophages had the highest diagnostic value to diagnose MPE, with a high sensitivity of 88.2% and a high specificity of 100%.
The parameters including positive predictive value, negative predictive value, positive likelihood ratio and negative likelihood ratio are also important for diagnostic analysis. The positive likelihood ratio value of CD206+CD14+ macrophages is infinite, which means that if CD206+CD14+ macrophages determination is positive, there was no possibility that the patient had benign pleural effusion. The negative likelihood ratio value is 0.1, which means that if CD206+CD14+ macrophages determination is negative, the chance that this patient has MPE is 10%. What’s more, CD206+CD14+ macrophages also have a high positive predictive value of 100.0% and a high negative predictive value of 86.7%, respectively. Our current data indicate that CD206+CD14+ macrophages can be used as a specific marker to rule out MPE from benign pleural effusion.
It should be aware that the detection of CD206+CD14+ macrophages based on flow cytometry is simple and rapid. Based on the high sensitivity and high specificity of CD206+CD14+ macrophages for MPE, it is recommended that the detection of CD206+CD14+ macrophages is performed in routine clinical practice. We will further analyze the relationship between the high percentage of CD206+CD14+ macrophages and the overall survival of patients with MPE. Targeting tumor-associated macrophages have been reported to be a new and reasonable anticancer therapy (22-26). It is especially important to figure out the phenotypes and subsets of macrophages in MPE, and CD206+CD14+ macrophages may be a novel therapeutic target for treating patients with MPE.
Our study had some limitations. First, the sample size enrolled in our study was relatively small, with 34 patients with MPE and 26 with benign pleural effusion. Therefore, larger populations are required to confirm the value of this approach. Second, MPE analyzed in our study were all derived from lung cancer. Benign pleural effusions were derived from tuberculosis, pneumonia and heart failure. Pleural effusion induced by other types of malignant and benign disease will be analyzed and the diagnostic value of CD206+CD14+ macrophages for MPE will be confirmed in our further studies.
Taken together, our data presented that the percentages of CD14+, CD86+CD14+ and CD206+CD14+ macrophages are significantly increased in MPE compared with those in benign pleural effusion. They can be used as diagnostic indicators to discriminate MPE from benign pleural effusion. In addition, the diagnostic performance of CD206+CD14+ macrophages is more accurate than those of CD14+ and CD86+CD14+ macrophages.
Acknowledgments
Funding: This work was supported in part by grants from National Natural Science Foundation of China (No. 31470883, No. 81730046 and No. 31700790), Beijing Municipal Administration of Hospitals’ Mission Plan (No. SML20150301), Beijing Nova program (Z171100001117015), and Beijing Talents Foundation (2017000021223ZK38).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the ethics committee of Beijing Chao-Yang Hospital (No. 2018-Ke-195), and all patients signed the informed consent according to the approved guideline.
References
- Kastelik JA. Management of malignant pleural effusion. Lung 2013;191:165-75. [Crossref] [PubMed]
- Egan AM, McPhillips D, Sarkar S, et al. Malignant pleural effusion. Qjm 2014;107:179-84. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. [Crossref] [PubMed]
- Maskell NA, Butland RJ. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 2003;58 Suppl 2:ii8-17. [Crossref] [PubMed]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013;496:445-55. [Crossref] [PubMed]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010;11:889-96. [Crossref] [PubMed]
- Dong P, Ma L, Liu L, et al. CD86+/CD206+, Diametrically Polarized Tumor-Associated Macrophages, Predict Hepatocellular Carcinoma Patient Prognosis. Int J Mol Sci 2016;17:320. [Crossref] [PubMed]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29-36. [Crossref] [PubMed]
- Loddenkemper R. Thoracoscopy - state of the art. Eur Respir J 1998;11:213-21. [Crossref] [PubMed]
- Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer 2006;54:1-9. [Crossref] [PubMed]
- Zhai K, Wang W, Wang Y, et al. Diagnostic accuracy of tumor markers for malignant pleural effusion: a derivation and validation study. J Thorac Dis 2017;9:5220-9. [Crossref] [PubMed]
- Yang Y, Liu YL, Shi HZ. Diagnostic Accuracy of Combinations of Tumor Markers for Malignant Pleural Effusion: An Updated Meta-Analysis. Respiration 2017;94:62-9. [Crossref] [PubMed]
- Skalski JH, Astoul PJ, Maldonado F. Medical Thoracoscopy. Semin Respir Crit Care Med 2014;35:732-43. [Crossref] [PubMed]
- Wang Z, Wu YB, Xu LL, et al. Diagnostic value of medical thoracoscopy in malignant pleural effusion induced by non-Hodgkin's lymphoma. Oncol Lett 2017;14:8092-9. [PubMed]
- Wu YB, Xu LL, Wang XJ, et al. Diagnostic value of medical thoracoscopy in malignant pleural effusion. BMC Pulm Med 2017;17:109. [Crossref] [PubMed]
- Kinouchi M, Miura K, Mizoi T, et al. Infiltration of CD14-positive macrophages at the invasive front indicates a favorable prognosis in colorectal cancer patients with lymph node metastasis. Hepatogastroenterology 2011;58:352-8. [PubMed]
- Dai K, Huang L, Sun X, et al. Hepatic CD206-positive macrophages express amphiregulin to promote the immunosuppressive activity of regulatory T cells in HBV infection. J Leukoc Biol 2015;98:1071-80. [Crossref] [PubMed]
- Xu L, Zhu Y, Chen L, et al. Prognostic value of diametrically polarized tumor-associated macrophages in renal cell carcinoma. Ann Surg Oncol 2014;21:3142-50. [Crossref] [PubMed]
- Chen Y, Liang B, Zhao YJ, et al. Transcription expression and clinical significance of vascular endothelial growth factor mRNA and endostatin mRNA in pleural effusions of patients with lung cancer. Diagn Cytopathol 2012;40:287-91. [Crossref] [PubMed]
- Eltorgoman AE, Badr E, Kombr Y, et al. Pleural fluid DNA integrity index as a diagnostic marker of malignant pleural effusion. Br J Biomed Sci 2017;74:148-51. [Crossref] [PubMed]
- Wang F, Yang L, Gao Q, et al. CD163+CD14+ macrophages, a potential immune biomarker for malignant pleural effusion. Cancer Immunol Immunother 2015;64:965-76. [Crossref] [PubMed]
- DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011;1:54-67. [Crossref] [PubMed]
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell 2015;27:462-72. [Crossref] [PubMed]
- Guerriero JL. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends Mol Med 2018;24:472-89. [Crossref] [PubMed]
- Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014;25:846-59. [Crossref] [PubMed]
- Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013;73:1128-41. [Crossref] [PubMed]


