The characteristics of coronary-pulmonary artery fistulas and the effectivity of trans-catheter closure: a single center experience
Introduction
Coronary artery fistula (CAF) refers to the abnormal communications between coronary arteries and cardiac chambers, coronary sinus, pulmonary artery and vena cava. It is a rare heart malformation, present in 0.002% of the general population and represent 0.13% of congenital coronary anomalies, present in 0.002% of the general population and represent 0.13% of congenital coronary anomalies (1,2). While, the coronary-pulmonary artery fistula (CPF) refers to a rarer heart malformation of abnormal connection between the coronary artery and the pulmonary artery, and has been reported in only 17% of CAF cases (3-5). Previous study reported that the symptoms included fatigue, dyspnea on exertion and angina depending on the size and localization of CPFs and the coronary flow reserve. Coronary angiography is the "gold standard" for the diagnosis of CPF, however, with the development of CT imaging technology, the sensitivity and specificity of cardiac CTA in diagnosing CPF are significantly improved (3,4,6,7). Although the diagnosis rate of CPF is significantly improved, the management is still debated and controversial, especially in patients without symptoms. Moreover, because of the low morbidity, recommendations are always based on small retrospective series or few case reports. In addition, the choice between trans-catheter closure (TCC) of CPF and surgical intervention is still controversial (1,8-10). At present, coil embolization is becoming the first choice for CPFs, especially for patients whose anatomy is favorable for the procedure, including those with a single narrow drainage site, a proximal fistula origin, an absence of multiple fistulas or large branch vessels, and/or an absence of concomitant cardiac disorders (11,12). Therefore, we retrospectively reviewed 43 cases of CPFs in the Cardiology Department of Zhongshan Hospital to investigate the characteristics of CPF, and evaluate the effectivity of TCC for CPFs.
Methods
Patients and data collection
Patients with CPF who were hospitalized in the cardiology department during the period of 2008–2015 were retrospectively reviewed and enrolled according to the inclusion criteria. The inclusion criteria were as follows: (I) definite diagnosis of CPF; (II) not complicated with coronary atherosclerosis disease, cardiomyopathy, and other congenital heart diseases. The data of clinical manifestations, physical signs, electrocardiogram (ECG), echocardiography, coronary CTA, coronary angiography and intervention procedure were collected. The telephone follow-up was conducted to evaluate the prognosis.
This study was approved by the Institutional Ethics Review Board (Zhongshan Hospital, Fudan University Review Board B2016-045). All participants provided written informed consent prior to the start of the study.
Statistical analysis
Normally distributed continuous variables were expressed as means ± standard deviation (SD), otherwise, median (q25, q75) was applied. The categorical variables were expressed as number (percentages). For continuous variables, differences between groups were evaluated by t test for normally distributed values; otherwise, the Mann–Whitney U test was applied. For categorical variables, differences between groups were evaluated with the chi-square test. All probability values shown are 2 sided, and a probability level of P<0.05 was considered significant. Analysis was performed using the software package SPSS, version23 (SPSS Inc., Chicago, IL, USA).
Results
Epidemiological data
Forty-three patients with CPF who were admitted in the cardiology department during the period of 2008–2015 were eligible for the inclusion criteria. All patients were not complicated with coronary atherosclerosis disease, cardiomyopathy, and other congenital heart diseases. There were 21 males (48.84%) and 22 females (51.16%), with an average age of 54.2 years. Nineteen cases (44.19%) were coupled with hypertension, 6 cases (13.95%) with diabetes, 12 cases (27.91%) with hyperlipidemia.
Clinical symptoms and physical signs
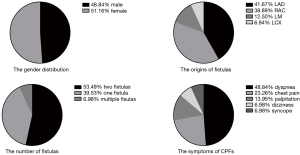
The most common presenting complaint was dyspnea/shortness of breath (n=21, 48.84%), followed by chest pain (n=10, 23.26%), palpitation (n=6, 13.95%), dizziness (n=3, 6.98%), and syncope (n=3, 6.98%). The most common physical examination finding was a murmur (n=6, 14%). Although most CPFs are asymptomatic and free of physical signs owing to the small size of the fistula, patients with larger CPFs are initially referred for evaluation of an unexplained loud continuous heart murmur, which is best heard in the second intercostal space, left of the sternum, in a crescendo-decrescendo pattern that is continuous throughout systole and diastole. The statistical pie diagram of the gender distribution, the symptoms of CPF, the number of fistulas, and the origin of fistulas are shown in Figure 1.
Laboratory examination data
No significant abnormalities were found in blood routine test, liver and kidney function, electrolyte, CRP, myocardial enzyme, NT-proBNP and other significant indexes. While, 19 cases (44.18%) were coupled with hypertriglyceridemia, 15 cases (34.88%) with high density lipoprotein <1.04 mmol/L, 6 cases (13.95%) with low density lipoprotein >3.2 mmol/L, 6 cases (13.95%) with HbA1c >6.0%.
ECG
Among the 43 cases, 29 cases (67.44%) displayed normal ECG and 8 cases (18.60%) were coupled with ST-T changes (mostly in II, III, AVF lead with depressed ST segment, low and flat T wave), 5 cases (11.63%) with showed sinus bradycardia, 3 cases (6.98%) combined with atrial and ventricular premature beats. Moreover, atrial fibrillation, Wolff-Parkinson-White syndrome, and I degree atrioventricular block were found in 1 case respectively.
Echocardiography
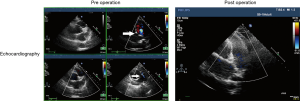
Echocardiography examination was performed in 39 patients, and the CPFs were accurately diagnosed in 19 patients (48.72%). There were 8 patients (20.51%) coupled with dilated left atrium (>40 mm) and 3 (7.69%) patients with increased pulmonary artery pressure (>40 mmHg), however, the ejection fraction, left ventricular end diameter and diastolic diameter were all in normal range in the 39 patients. The representative echocardiography images of CPFs are shown in Figure 2.
Cardiac CTA
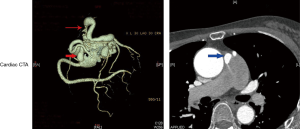
Twenty-four patients underwent coronary CTA examination and were all accurately diagnosed. The CPFs appeared as an abnormal contrast blush, which is also referred to as the contrast shunt sign, in a relatively less-opacified pulmonary trunk or as a well-visualized fistulous tract between the coronary artery and pulmonary trunk. The representative coronary CTA images of CPFs are shown in Figure 3.
Coronary angiography and TCC of CPF
All the 43 patients underwent coronary angiography before the TCC of CPF. A total of 72 fistulous tracts were founded in the 43 patients. According to the number of fistulas, 17 cases (39.53%) were coupled with single fistula, 23 cases (53.49%) with two fistulas, and 3 cases (6.98%) with multiple (n≥3) fistulas, indicating that single or two coronary fistulas are far more common than multiple coronary fistulas. Just as previous studies reported (3,7), CAF may arise from any coronary arteries, but more often originate from right coronary artery (RCA) and left anterior descending (LAD) artery, and the left circumflex artery (LCX) is rarely involved. In the present study, 30 fistulas (41.67%) involved the LAD, 28 fistulas (38.89%) involved the RCA, 9 fistulas (12.50%) involved the left main trunk (LM), and 5 fistulas (6.94%) involved the LCX. Most of the fistulous tracts originated within the proximal one-third of the coronary arteries, only 6 fistulous tracts (8.33%) originated from the distal segment of the coronary arteries. The size of the fistula arranged from 1 to 8 mm, with an average of 3.45 mm. The majority (n=64, 88.89%) of CPFs drain into the pulmonary trunk rather than into other segmental pulmonary arteries.
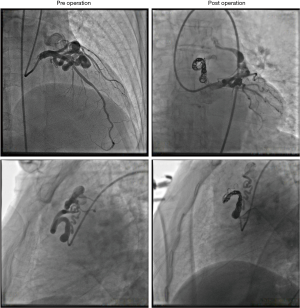
Thirty-five patients (81.40%) with 63 fistulas (87.50%) were successfully treated by percutaneous transcatheter closure. Because of the failure of entering the distal end of fistulas, 5 fistulas (6.94%) in 5 patients (11.63%) failed to be closed. Two patients (4.65%) gave up further treatment before the operation. In one case, the coil ejected out of the fistula after the detachment and migrated to the small branch of internal iliac artery. Several methods, including the usage of catcher, were conducted but still failed to take out the migrated coil. Because the vascular branch was small, we gave up the catchment of migrated coil. No obvious complication of the migrated coil was founded in the follow-up. The representative operative coronary angiography images are shown in Figure 4.
The follow-up
The patients’ post-procedure courses were uneventful, and no major complications, such as coil migration, distal coronary embolization, acute myocardial infarction, malignant arrhythmia, and acute heart failure, were observed. Thirty-eight patients accepted the 6-month follow-up, 36 patients (94.74%) were asymptomatic and 2 patients (5.26%) with palpitation.
Discussion
CPFs are relatively rare coronary artery anomalies with a prevalence approximately 1%, always occasionally and incidentally found in the adult coronary angiography. The most widely accepted embryologic explanation for a CPF is the Hackensellner involution-persistence hypothesis (13). According to this theory, among the six branches of the truncus, only two branches from the aortic sinus remain to form the coronary arteries, while the rest four of the branches involute. The abnormal persistence of the branch from the pulmonary sinus, which is otherwise involuted, connects to the normal branches from the aortic sinus coronary arteries to give rise to a CPF.
While dyspnea and angina are the most prevalent among reported symptoms, atrial arrhythmia, pulmonary hypertension, congestive heart failure, presence of aneurysms, rupture or thrombosis of the fistula, and endocarditis, are also reported (14-17). The severity of the symptoms was always related with the number and size of the fistulas. Patients with more fistulas, larger fistulous tract diameter and more severe left-to-right shunt are always coupled with more obvious and severer clinical manifestations. In the present study, 3 patients who complained of syncope all had 2 large fistulas with an average diameter of 5mm. The murmurs were often not obvious. In the current study, the murmurs can be heard in only 6 patients at the 2 or 3 intercostal area.
Due to the asymptomatic nature of CPFs, most diagnosis of CPFs is incidental findings during routine examinations by noninvasive imaging techniques. The most widely employed techniques were coronary CTA and echocardiography, while the sensitivity and specificity of coronary CTA are much more satisfactory. The abnormal contrast blush, which is also referred to as the contrast shunt sign, in a relatively less-opacified pulmonary trunk or as a well-visualized fistulous tract between the coronary artery and pulmonary trunk is the most typical CTA feature of CPF. Multiplanar reconstruction with 3D volume-rendered imaging could yield excellent anatomic information, including the origin, course, and drainage site of CPFs—even in cases of complex anomalies. Therefore, the coronary CTA are valuable tools for the cardiac physician to evaluate the origin, course, and drainage site of CPFs preoperatively and make the best management of fistulas (18,19).
Due to the low prevalence of CPFs, the optimal treatment strategy remains unclear, and there are no established therapeutic guidelines available. The management strategy for patients with CPFs always depends on the size of the fistula, presence of symptoms, anatomy of the fistula, patient’s age, and presence of associated cardiovascular abnormalities. Small asymptomatic CPFs are often treated with conservative medical therapy. According to ACC/AHA guidelines for the management of adults with congenital heart disease, interventional management is a class I recommendation for large CAFs, regardless of the symptoms, and small to moderate-size fistulas with symptoms, including myocardial ischemia, arrhythmia, ventricular dysfunction, and endarteritis (20). Treatment options include surgical ligation and percutaneous transcatheter closure. While in fact, many retrospective reviews concluded that complications involved in surgical correction might outweigh the risk of CPFs complications (21,22). Percutaneous transcatheter closure is a noninvasive alternative for treatment of CPFs and more beneficial than surgical approaches for eligible CPF cases. Transcatheter techniques do not require median sternotomy or cardiopulmonary bypass, thus limiting potential iatrogenic complications. Transcatheter closure is also a less expensive procedure with decreased morbidity, decreased recovery time, and better cosmetic results. Therefore, the tans-catheter closure is becoming the first choice for CPFs. The detachable coil was the most widely employed device for the tans-catheter closure of CPFs. Compared with other devices, it has the advantages of good controllability, safety, less expensive price, easy deployment, so that the residual leakage or recanalization of the fistulas were significantly reduced (1,8,11,12).
There are many factors, such as the size, origin site, and tortuosity of the fistulas, augment the difficulties of coil embolization of the fistulas. Among these factors, the tortuosity of the vessel is an important obstacle, because the guidewire and the microcatheter was hardly advanced to the distal segment of the tortuous vessel, which always resulted in the failure of coil embolization of the fistulas. In patients with extremely tortuous fistula, multiple fistulas or additional cardiac disease requiring surgery, surgical correction is usually considered. However, surgical procedures such as cardiopulmonary bypass and median sternotomy carry additional risks. Therefore, new techniques are in great needed to improve the success rate of coil embolization for complex CPFs.
Although percutaneous transcatheter closure is less invasive than surgery, it can also result in dangerous complications such as residual leakage or recanalization of the fistulas, coil migration and distal embolization, especially in patients with a large fistula and a high-flow shunt. Coil migration into a pulmonary artery can cause life-threatening complications, such as pulmonary embolism. In order to avoid the above complications, it is necessary to evaluate the origin, course, and drainage site of the fistulas carefully and to select coils of optimal size and release them only at the curve in the vessel. The coil size is always 10–20% more than the artery to be occluded. Another serious complication after percutaneous intervention is thromboembolism, which is also a common cause of sudden death after operation. Therefore, antiplatelet therapy was always required after the tans-catheter closure. However, there was no guideline about the period of antiplatelet therapy. In the present study, the patients accepted 3-month antiplatelet therapy after the operation, and no thromboembolism complications (such as stroke and pulmonary embolism) were found.
Conclusions
In summary, CPFs are relatively rare coronary artery anomalies. Patients with more fistulas, larger fistulous tract diameter and more severe left-to-right shunt are always coupled with more obvious and severer clinical manifestations. The trans-catheter coil embolization is an effective method for the closure of CPFs. New techniques and devices are still needed to overcome the obstacles obstructing the coil embolization of complex CPFs.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Ethics Review Board (Zhongshan Hospital, Fudan University Review Board B2016-045). All participants provided written informed consent prior to the start of the study.
References
- Cebi N, Schulze-Waltrup N, Fromke J, et al. Congenital coronary artery fistulas in adults: concomitant pathologies and treatment. Int J Cardiovasc Imaging 2008;24:349-55. [Crossref] [PubMed]
- Yamanaka O, Hobbs RE. Coronary-Artery Anomalies in 126,595 Patients Undergoing Coronary Arteriography. Cathet Cardiovasc Diagn 1990;21:28-40. [Crossref] [PubMed]
- Kim MS, Jung JI, Chun HJ. Coronary to pulmonary artery fistula: morphologic features at multidetector CT. Int J Cardiovasc Imaging 2010;26:273-80. [Crossref] [PubMed]
- Zhang LJ, Zhou CS, Wang Y, et al. Prevalence and types of coronary to pulmonary artery fistula in a Chinese population at dual-source CT coronary angiography. Acta Radiol 2014;55:1031-9. [Crossref] [PubMed]
- Said SAM, Lam J, van der Werf T. Solitary coronary artery fistulas: a congenital anomaly in children and adults. A contemporary review. Congenit Heart Dis 2006;1:63-76. [Crossref] [PubMed]
- Shabestari AA, Akhlaghpoor S, Fatehi M. Findings of bilateral coronary to pulmonary artery fistula in 64-multislice computed tomographic angiography: Correlation with catheter angiography. J Comput Assist Tomogr 2008;32:271-3. [Crossref] [PubMed]
- Scandura S, Cammalleri V, Ronsivalle G, et al. Multimodality imaging of a left main coronary artery-to-pulmonary artery fistula. J Cardiovasc Med (Hagerstown) 2017;18:704-5. [Crossref] [PubMed]
- Ata Y, Turk T, Bicer M, et al. Coronary arteriovenous fistulas in the adults: natural history and management strategies. J Cardiothorac Surg 2009;4:62. [Crossref] [PubMed]
- Verdini D, Vargas D, Kuo A, et al. Coronary-Pulmonary Artery Fistulas: A Systematic Review. J Thorac Imaging 2016;31:380-90. [Crossref] [PubMed]
- Armsby LR, Keane JF, Sherwood MC, et al. Management of coronary artery fistulae. J Am Coll Cardiol 2002;39:1026-32. [Crossref] [PubMed]
- Kabbani Z, Garcia-Nielsen L, Lozano ML, et al. Coil embolization of coronary artery fistulas. A single-centre experience. Cardiovasc Revasc Med 2008;9:14-7. [Crossref] [PubMed]
- Issa M, Berzingi C, Alkhouli M. Resolution of exertional angina after coil embolization of coronary to pulmonary artery fistula. J Card Surg 2018;33:308-9. [Crossref] [PubMed]
- Heifetz SA, Robinowitz M, Mueller KH, et al. Total Anomalous Origin of the Coronary-Arteries from the Pulmonary-Artery. Pediatr Cardiol 1986;7:11-8. [Crossref] [PubMed]
- Said SAM. Characteristics of Congenital Coronary Artery Fistulas Complicated with Infective Endocarditis: Analysis of 25 Reported Cases. Congenit Heart Dis 2016;11:756-65. [Crossref] [PubMed]
- Vieira MS, Antunes N, Anjo D, et al. Coronary artery fistula presenting as unstable angina. Rev Port Cardiol 2013;32:165-7. [Crossref] [PubMed]
- Abdul Jabbar A, Patel A, Marzlin N, et al. Internal mammary artery-to-pulmonary vasculature fistula: Systematic review of case reports. Vasc Med 2017;22:426-31. [Crossref] [PubMed]
- Yun G, Nam TH, Chun EJ. Coronary Artery Fistulas: Pathophysiology, Imaging Findings, and Management. Radiographics 2018;38:688-703. [Crossref] [PubMed]
- Lee CM, Song SY, Jeon SC, et al. Characteristics of Coronary Artery to Pulmonary Artery Fistula on Coronary Computed Tomography Angiography. J Comput Assist Tomogr 2016;40:398-401. [Crossref] [PubMed]
- Okwuosa TM, Gundeck EL, Ward RP. Coronary to pulmonary artery fistula - Diagnosis by transesophageal echocardiography. Echocardiography 2006;23:62-4. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e143-e263. [Crossref] [PubMed]
- Liao PC, Hsieh SR, Hsieh YC, et al. Surgical Ligation of Bilateral Large Coronary Artery Fistula to Pulmonary Artery. JACC Cardiovasc Interv 2015;8:e203-4. [Crossref] [PubMed]
- Kamiya H, Yasuda T, Nagamine H, et al. Surgical treatment of congenital coronary artery fistulas: 27 years experience and a review of the literature. J Card Surg 2002;17:173-7. [Crossref] [PubMed]