Approach to chronic cough: the neuropathic basis for cough hypersensitivity syndrome
Introduction
Cough is a chronic symptom not only found in patients with a range of respiratory and non-respiratory conditions but also in apparently “normal” individuals. Chronic cough is very prevalent in the community (9-33% according to various studies) and may be increasing in association with increasing environmental pollution to which we are increasingly exposed to (1-3). An acute cough is a cough that lasts for less than 3 weeks and is usually caused by an upper respiratory tract virus infection such as the common cold. A cough that persists for more than 8 weeks is termed “chronic” although it may have been persistent for much longer periods before presentation to the doctor. Chronic cough can persist for months and years and remains a difficult problem to manage because of our poor understanding of why a cough can be so persistent and also by the lack of effective antitussive therapies. However, there has been recent progress both in our diagnostic approach and in our general understanding of the process of chronic cough over the recent years.
Approach to the cough patient in the clinic
The recognition of chronic cough as being an important increasing problem has led to the publication of national guidelines for the diagnosis and management of cough in many countries in the world (4-7). These guidelines correctly propose an anatomico-diagnostic approach to the potential causes of the chronic cough, and treating the cause. The most common conditions associated with causing chronic cough, with a normal chest radiograph, include the corticosteroid-responsive eosinophilic airway diseases (asthma, cough variant asthma and eosinophilic bronchitis), and a range of conditions typically associated with an inhaled corticosteroid-resistant cough including gastro-oesophageal reflux disease (GORD) and the post-nasal drip syndrome or rhinosinusitis (Table 1). There are some geographical variants. In China, a recent survey of chronic cough patients presenting to a hospital respiratory clinic showed a distribution of associated diagnoses as described in Europe or USA (8). However, in Japan, atopic cough and sinobronchial disease are more commonly diagnosed, while GORD is much less so (9,10).

Full table
Asthma and eosinophilic conditions
Chronic cough in asthma is not always associated with airflow obstruction, wheezing or dyspnoea. Asthma can predominantly present with cough, which is often nocturnal; the diagnosis is supported by the presence of bronchial hyper-responsiveness (11). Cough is often the symptom most reported by patients with chronic asthma, despite achieving good asthma control with inhaled corticosteroids. Other related conditions have been described: cough-variant asthma, atopic cough, and eosinophilic bronchitis. Cough-variant asthma presents with a dry cough, often nocturnal, without other symptoms of asthma; it is characterised by bronchial hyper-responsiveness, and eosinophilic inflammation in sputum, bronchoalveolar lavage fluid, or airway submucosa (12). Atopic cough is an isolated chronic cough characterised by an atopic background, eosinophilia in sputum, cough hypersensitivity, normal pulmonary function and airway responsiveness (13). The clinical condition of eosinophilic bronchitis is characterised by a troublesome cough without other symptoms of asthma or bronchial hyper-responsiveness, but with increased numbers of eosinophils in the sputum (14,15). Pathological features of the airway submucosa are similar to those of asthma (16).
Eosinophilic-associated cough is usually controlled by inhaled corticosteroids, implying a role for inflammatory factors. Although patients with classic asthma do not usually have an enhanced cough reflex, patients with cough-variant asthma might do so (17), as do patients with eosinophilic bronchitis and atopic cough. Inflammatory cells, such as eosinophils, have been implicated, since corticosteroids reduce eosinophilic inflammation and also inhibit cough. A case-report of hypereosinophilic syndrome also supports a direct effect of eosinophils on the cough reflex (18). Sensitivity to capsaicin in asthmatic patients who are allergic to birch pollen, increases during the birch pollen season (19), suggesting that allergic inflammation can trigger neurogenic mechanisms of sensitisation.
Chronic cough is now being considered as an important symptom of chronic obstructive pulmonary disease (COPD) that has been relatively undervalued so far. It is associated with an augmented capsaicin cough reflex (17,20). Cough can be the earliest sign of an impending exacerbation and can be the worst symptom experienced by the COPD patient. More importantly, cough impacts adversely on patients’ health status and forms an important component of recently validated quality of life instruments (21).
Gastro-oesophageal reflux disease
GORD encompasses symptoms or complications such as heart burn, chest pain, sour taste or regurgitation, and also a chronic persistent cough. Direct aspiration of gastric contents into the larynx and upper airways that could directly stimulate cough receptors and increases in tracheal acidity have been recorded during episodes of reflux (22), but the majority of coughs in GORD does not coincide with an acid reflux episode (23). In fact, episodes of reflux could precede or occur after a cough (24). The fact that very effective pharmacological control of gastric acid with proton pump inhibitors are not usually effective in controlling cough associated with GORD would suggest that acid reflux alone does not cause the cough (25). The role of non-acid reflux mechanisms still need to be excluded and the potential use of fundoplasty for GORD-associated cough is unclear. It is possible that that gastric reflux contents may be related to the cough hypersensitivity syndrome in that there is a greater sensitivity of ‘cough’ receptors caused by mucosal damage at the level of the oesophagus. Laryngopharyngeal reflux is an extraesophageal variant of GORD recognised by otorhinolaryngologists, and the symptoms often include hoarseness, chronic cough, sore throat, globus pharyngeus, and throat-clearing (26).
Post-nasal drip
Post-nasal drip (“nasal catarrh”) is characterised by a sensation of nasal secretions or of a “drip” at the back of the throat, accompanied very often by a frequent need to clear the throat (“throat-clearing”) associated with nasal discharge or nasal stuffiness. This symptom of throat clearing could also be considered as a symptom of “hypersensitivity”. The term upper airway cough syndrome is proposed as an alternative to stress the association of upper airways disease with cough (27). The pathogenesis of cough in the post-nasal drip syndrome may be related to the direct pharyngeal, laryngeal or sublaryngeal stimulation by the mucoid secretions from the rhinosinuses which contain inflammatory mediators that could induce cough. Specific treatment of rhinosinusitis with an antihistamine, an anticholinergic and topical corticosteroids provided only partial relief of the accompanying cough (28).
“Idiopathic” cough or cough of unknown cause
Table 1 summarises the various types of chronic cough. Out of those mentioned, the chronic cough associated with conditions that are treated that does not lead to resolution of cough and the chronic cough not associated with any conditions and unresponsive to any treatments remain the most difficult to manage. These patients are often labelled as “idiopathic” cough and constitute a significant proportion of any cough clinic (1). Therefore, such a label is usually applied to a chronic cough that is not apparently associated with known causes of cough, established either through intensive investigation to exclude these causes or through trial of therapy of these causes. The initiating cause of the cough may have disappeared, but its effect on enhancing the cough reflex may be more prolonged. An example could be the transient appearance of an upper respiratory tract virus infection or an exposure to toxic fumes that causes prolonged damage of the airways mucosa. These insults may have induced inflammatory neuropathic changes in the sensory nerves. The repetitive mechanical and physical effects of coughing bouts on airway cells could activate the release of various chemical mediators that could enhance chronic cough through inflammatory mechanisms (29), providing a positive feed-forward system for cough persistence. Changes in the upper airways of inflammation and tissue remodelling may be induced by various causes associated with cough or by the act of coughing itself that could lead to an enhanced cough reflex, that in turn is responsible for maintaining cough. The cough becomes “idiopathic” when the primary inciting cause has resolved while cough remains persistent.
Symptoms associated with chronic cough
Patients with chronic cough often complain of a persistent tickling or irritating sensation in the throat (feeling of an itch) or a choking sensation, and it is sometimes felt in the chest, often leading to paroxysms of coughing. Other symptoms associated with chronic cough patients include an irritation in the throat or chest, with clearing of the throat, an irritation in the chest associated with chest tightness, hoarse voice and dysphonia, vocal cord dysfunction symptoms, a feeling of globus, dysphagia and acid reflux symptoms (Table 2). Coughing induced by reflex mechanisms, as distinct from voluntary or habit coughing, is often associated with unpleasant sensation in the chest or throat; however this is not always present, especially with conditions in the lower airways involving, for example, excessive mucus. The terms used to describe the sensations are multiple, and include irritation, rawness, even pain (30). Unpleasant sensations related to cough may be localized in the throat or in the chest. Other respiratory sensations, such as air-hunger, sense of effort and sense of lung volume are not usually associated with cough. Triggers such as changes in ambient temperature, taking a deep breath, laughing, talking over the phone for more than a few minutes, cigarette smoke, aerosol sprays, perfumes or eating crumbly dry food are common.
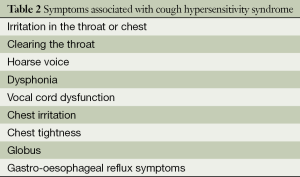
Full table
Urge-to-cough is a distinct sensation that, with increasing levels of cough stimulation, has a lower threshold and occurs before the cough itself (31). While urge-to-cough has no particular anatomical location, unpleasant sensations related to cough may also be felt in the chest or the throat. The urge-to-cough has been shown to be a sensory measure of this sensation of tickling or irritation that is induced at lower concentrations of inhaled capsaicin than those that will elicit a cough reflex (32).
Cough hypersensitivity syndrome
The combination of irritation in the throat or upper chest representative of laryngeal or pharyngeal or upper airway paresthesiae, of cough triggered by non-tussive stimulus such as talking, laughing termed allotussia, and of increased cough sensitivity to inhaled stimuli and number of triggers termed hypertussia (Table 3) suggest a disorder of airway sensory neural function that has led to the introduction of the term chronic “cough hypersensitivity syndrome” to describe chronic cough (33). This terms proposes that this disordered sensory neural function (and hence the cough hypersensitivity which underlies chronic cough in general) reflects an underlying sensory neuropathy. Recently, a Task Force of the European Respiratory Society has defined the cough hypersensitivity syndrome as “a clinical syndrome characterised by troublesome coughing often triggered by low levels of thermal, mechanical, or chemical exposure” (34). The Laryngeal Hypersensitivity Questionnaire has been developed recently as a simple, non-invasive tool to measure laryngeal paresthesia in patients with laryngeal conditions such as chronic cough, paradoxical vocal fold movement (vocal cord dysfunction), muscle tension dysphonia, and globus pharyngeus (35), conditions which can be considered to be part of the cough hypersensitivity syndrome (36).

Full table
Mechanisms of cough hypersensitivity
These potential mechanisms have recently been reviewed (37). Sensory afferent nerves are susceptible to sensitization by neuroactive molecules such as nerve growth factor through changing the activation profiles of cough afferent nerves, and facilitating afferent encoding signals in response to irritant stimuli (38-40). Furthermore, sensitization may be related to altered expression of ion channels and other receptor molecules, including TRPV1 which regulates afferent nerve excitability to many chemical stimuli (41). Cough hypersensitivity can also be induced when normal afferent signals are augmented by central events through the interaction of different subsets of afferent neurons in the brainstem (42). Neuropeptide expressed in airway nociceptors and airway mechanosensors can reduce the cough reflex threshold through the convergence onto common second order neurons in the brainstem (43). This process may lead to the amplification of the incoming signals that are received by the brainstem cough network. For example, cigarette smoke exposure in primates can lead to increased excitability of second order neurons in the brainstem receiving inputs from the airways, an effect prevented by blocking the neuropeptide, substance P (44). Induction of neuropeptide expression by airway mechanosensors following antigen or viral exposure (39,40) may negate the need for these convergent inputs to cause central sensitization. Neurons in the medulla receiving inputs from airway afferents also project to many subcortical nuclei in the pons, thalamus, hypothalamus, midbrain and amygdala. Using functional brain imaging, neural activation pathways have been shown during voluntary cough and the urge-to-cough (45,46).
Inflammatory factors and neurogenic mechanisms
Chronic cough has been associated with airway inflammation and remodelling. Damaged bronchial epithelial, basement membrane thickening and a chronic inflammatory infiltrate have been described in airway biopsies from patients with unexplained chronic cough (47-49). In bronchial biopsy studies of chronic cough, an increase in the number of mast cells together with features of airway wall remodelling characterised by an increase in vascular profiles, subepithelial fibrosis and hyperplasia of goblet cells has been reported (47). Other studies have reported increased mast cells and neutrophils in bronchoalveolar lavage fluid, with increased levels of various inflammatory biomarkers including histamine, prostaglandin D2 and E2, TNFα and IL-8 in induced sputum samples (50-53). The expression of the growth factor, TGFβ, was increased in the airways of patients with chronic cough in relation to the degree of airway fibrosis as measured by the thickness of the subbasement membrane (53), and that could in turn lead to activation of the cough reflex.
Chronic eosinophilic conditions such as asthma, cough variant asthma and eosinophilic bronchitis frequently present as chronic cough and suppression of the eosinophilic inflammation improves cough, indicating a pathogenic role for eosinophils. Eosinophils co-localise with sensory airway nerves (54), which may lead to the release of mediators including eosinophil peroxidase and leukotrienes (55). The neuroinflammatory effects of these eosinophil derived mediators may provide a mechanism whereby cough reflex hypersensitivity may be maintained during airway inflammation (56). Inhalation of inflammatory mediators such as bradykinin and PGE2 by healthy volunteers upregulated the capsaicin cough response (57). Both mediators are known to indirectly sensitize airway neuronal responses to capsaicin presumably via activation of the TRPV1 channel by intracellular (protein kinase) pathways (58). Increased H+ ions in exhaled breath condensates from chronic cough patients has been reported, which together with the increase in TRPV1 receptors reported in the epithelial nerves of chronic cough patients (41,59) could form the basis for the increased cough.
There is evidence of airway neuronal activation reflected in the detection of elevated levels of substance P and neurokinin A in induced sputum samples obtained from asthmatic coughers (60). Furthermore, levels of the neuropeptide calcitonin-gene-related peptide (CGRP) measured in airway lavage samples from paediatric patients with chronic cough were positively correlated with capsaicin cough reflex sensitivity (61). CGRP expression is also reported to be increased in airway nerves from patients with chronic cough (62). Nerve growth factor (NGF) is released from a variety of airway cells including the bronchial epithelium and has important neuroinflammatory consequences which may be important in chronic cough. In diseases such as cryptogenic fibrosing alveolitis where cough is often an intractable problem airway levels of NGF are elevated (63). NGF may exert its action on airway sensory nerves via sensitization of the TRPV1 receptor. In primary cultured rat dorsal root ganglion neurones, NGF potentiated basal and capsaicin-induced expression of substance P and TRPV1 suggesting a mechanism for chronic nerve sensitization (64). In patients troubled with airway sensory hyperreactivity to scents and chemicals, typically manifest by bouts of coughing, higher levels of NGF have been detected in nasal secretions compared to healthy controls (65), although in bronchoalveolar lavage fluid obtained from chronic cough patients, the levels of NGF were not increased (53).
Respiratory viruses and cough: a neuropathic link?
Respiratory viral infections such as rhinoviruses or influenza viruses are typically accompanied by an acute cough, but this cough may persist for weeks or months in some patients. Experimental models of rhinovirus infection have demonstrated cough reflex hypersensitivity to chemical (66,67) and mechanical stimulation (68). The mechanisms by which these respiratory viruses can induce neuropathic changes are unknown but could certainly contribute to the cough hypersensitivity syndrome.
Evidence from recent antitussive therapies of a neuropathic cough
The recent use of drugs such as amitriptyline and gabapentin used to treat chronic pain as an antitussive also lends support to the concept of chronic cough being a neuropathic condition. In 12 patients treated with the antidepressant amitriptyline, 11 had prompt significant reduction of their cough (69). A prospective, randomized, controlled open trial comparing the effectiveness of amitriptyline versus codeine/guaifenesin for chronic cough with suspected post-viral vagal neuropathy showed that most subjects in the amitriptyline arm achieved a complete response while none of the codeine/guaifenesin group had a complete response (70). Indeed, ear-nose-throat specialists have long recognised chronic cough as a vagal neuropathic condition and have taken such an approach for a while. Gabapentin is another agent that has been used to treat neuropathic pain and has been shown to be effective in reducing cough in chronic cough patients in a randomised double-blind trial, suggesting that there is a central reflex sensitisation in refractory chronic cough (71). Gabapentin was also beneficial in chronic cough patients with laryngeal sensory neuropathy (72).
There is evidence that amitriptyline and gabapentin have central anti-nociceptive actions. Thus, relief from rectal pain by amitriptyline is associated with a reduction in pain-related responses in the anterior cingulate cortex in irritable bowel syndrome (73). Gabapentin reduces pain via an action on GABAergic neurotransmission or voltage gated ion channels in the spinal cord, midbrain, thalamus and/or sensory and insula cortices in the brain (74,75). Although gabapentin was effective in reducing cough in the chronic cough patients, it had no effect on capsaicin sensitivity arguing against a suppressive effect on cough reflex pathways. It is not excluded that amitriptyline and gabapentin may also have actions outside of the central nervous system, primarily by blocking the activation of peripheral afferent terminals.
Conclusions
The approach to management of chronic cough will still focus on diagnosing associated conditions and their treatments. The concept of cough hypersensitivity syndrome will help us understand the mechanisms underlying cough and will provide better antitussives to treat chronic cough.
Acknowledgements
This project was supported by NIHR Respiratory Biomedical Research Unit at the Royal Brompton NHS Foundation Trust and Imperial College London.
Disclosure: The author declares no conflict of interest.
References
- Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008;371:1364-74. [PubMed]
- Cullinan P. Persistent cough and sputum: prevalence and clinical characteristics in south east England. Respir Med 1992;86:143-9. [PubMed]
- Zemp E, Elsasser S, Schindler C, et al. Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). The SAPALDIA Team. Am J Respir Crit Care Med 1999;159:1257-66. [PubMed]
- Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007;29:1256-76. [PubMed]
- Gibson PG, Chang AB, Glasgow NJ, et al. CICADA: Cough in Children and Adults: Diagnosis and Assessment. Australian cough guidelines summary statement. Med J Aust 2010;192:265-71. [PubMed]
- Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006;129:1S-23S. [PubMed]
- Asthma Workgroup, Chinese Society Respiratory Diseases (CSRD), Chinese Medical Association. The Chinese national guidelines on diagnosis and management of cough (December 2010). Chin Med J (Engl) 2011;124:3207-19. [PubMed]
- Ma W, Yu L, Wang Y, et al. Changes in health-related quality of life and clinical implications in Chinese patients with chronic cough. Cough 2009;5:7. [PubMed]
- Committee for the Japanese Respiratory Society Guidelines for Management of Cough, Kohno S, Ishida T, et al. The Japanese Respiratory Society guidelines for management of cough. Respirology 2006;11:S135-86. [PubMed]
- Matsumoto H, Niimi A, Takemura M, et al. Prevalence and clinical manifestations of gastro-oesophageal reflux-associated chronic cough in the Japanese population. Cough 2007;3:1. [PubMed]
- Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med 1979;300:633-7. [PubMed]
- Niimi A, Amitani R, Suzuki K, et al. Eosinophilic inflammation in cough variant asthma. Eur Respir J 1998;11:1064-9. [PubMed]
- Fujimura M, Ogawa H, Nishizawa Y, et al. Comparison of atopic cough with cough variant asthma: is atopic cough a precursor of asthma? Thorax 2003;58:14-8. [PubMed]
- Gibson PG, Dolovich J, Denburg J, et al. Chronic cough: eosinophilic bronchitis without asthma. Lancet 1989;1:1346-8. [PubMed]
- Brightling CE, Ward R, Wardlaw AJ, et al. Airway inflammation, airway responsiveness and cough before and after inhaled budesonide in patients with eosinophilic bronchitis. Eur Respir J 2000;15:682-6. [PubMed]
- Brightling CE, Symon FA, Birring SS, et al. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol 2002;110:899-905. [PubMed]
- Doherty MJ, Mister R, Pearson MG, et al. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 2000;55:643-9. [PubMed]
- Chung KF, Hew M, Score J, et al. Cough and hypereosinophilia due to FIP1L1-PDGFRA fusion gene with tyrosine kinase activity. Eur Respir J 2006;27:230-2. [PubMed]
- Weinfeld D, Ternesten-Hasséus E, Löwhagen O, et al. Capsaicin cough sensitivity in allergic asthmatic patients increases during the birch pollen season. Ann Allergy Asthma Immunol 2002;89:419-24. [PubMed]
- Blanc FX, Macedo P, Hew M, et al. Capsaicin cough sensitivity in smokers with and without airflow obstruction. Respir Med 2009;103:786-90. [PubMed]
- Calverley PM. Cough in chronic obstructive pulmonary disease: is it important and what are the effects of treatment? Cough 2013;9:17. [PubMed]
- Jack CI, Calverley PM, Donnelly RJ, et al. Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastro-oesophageal reflux. Thorax 1995;50:201-4. [PubMed]
- Ours TM, Kavuru MS, Schilz RJ, et al. A prospective evaluation of esophageal testing and a double-blind, randomized study of omeprazole in a diagnostic and therapeutic algorithm for chronic cough. Am J Gastroenterol 1999;94:3131-8. [PubMed]
- Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology 2010;139:754-62. [PubMed]
- Smith JA, Houghton LA. The oesophagus and cough: laryngo-pharyngeal reflux, microaspiration and vagal reflexes. Cough 2013;9:12. [PubMed]
- Patel D, Vaezi MF. Normal esophageal physiology and laryngopharyngeal reflux. Otolaryngol Clin North Am 2013;46:1023-41. [PubMed]
- Pratter MR. Chronic upper airway cough syndrome secondary to rhinosinus diseases (previously referred to as postnasal drip syndrome): ACCP evidence-based clinical practice guidelines. Chest 2006;129:63S-71S. [PubMed]
- Macedo P, Saleh H, Torrego A, et al. Postnasal drip and chronic cough: An open interventional study. Respir Med 2009;103:1700-5. [PubMed]
- Heino M, Juntunen-Backman K, Leijala M, et al. Bronchial epithelial inflammation in children with chronic cough after early lower respiratory tract illness. Am Rev Respir Dis 1990;141:428-32. [PubMed]
- Widdicombe J. Lung afferent activity: implications for respiratory sensation. Respir Physiol Neurobiol 2009;167:2-8. [PubMed]
- Vovk A, Bolser DC, Hey JA, et al. Capsaicin exposure elicits complex airway defensive motor patterns in normal humans in a concentration-dependent manner. Pulm Pharmacol Ther 2007;20:423-32. [PubMed]
- Davenport PW, Bolser DC, Vickroy T, et al. The effect of codeine on the Urge-to-Cough response to inhaled capsaicin. Pulm Pharmacol Ther 2007;20:338-46. [PubMed]
- Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther 2011;24:267-71. [PubMed]
- Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014. [Epub ahead of print]. [PubMed]
- Vertigan AE, Bone SL, Gibson PG. Development and validation of the Newcastle laryngeal hypersensitivity questionnaire. Cough 2014;10:1. [PubMed]
- Vertigan AE, Bone SL, Gibson PG. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology 2013;18:948-56. [PubMed]
- Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respir Med 2013;1:414-22. [PubMed]
- Carr MJ, Hunter DD, Jacoby DB, et al. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med 2002;165:1071-5. [PubMed]
- Chuaychoo B, Hunter DD, Myers AC, et al. Allergen-induced substance P synthesis in large-diameter sensory neurons innervating the lungs. J Allergy Clin Immunol 2005;116:325-31. [PubMed]
- Myers AC, Kajekar R, Undem BJ. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol 2002;282:L775-81. [PubMed]
- Groneberg DA, Niimi A, Dinh QT, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 2004;170:1276-80. [PubMed]
- Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 2005;569:559-73. [PubMed]
- Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol 2002;283:R86-98. [PubMed]
- Sekizawa S, Joad JP, Pinkerton KE, et al. Distinct tachykinin NK(1) receptor function in primate nucleus tractus solitarius neurons is dysregulated after second-hand tobacco smoke exposure. Br J Pharmacol 2011;163:782-91. [PubMed]
- Mazzone SB, McLennan L, McGovern AE, et al. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med 2007;176:327-32. [PubMed]
- Mazzone SB, Cole LJ, Ando A, et al. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci 2011;31:2948-58. [PubMed]
- Niimi A, Torrego A, Nicholson AG, et al. Nature of airway inflammation and remodeling in chronic cough. J Allergy Clin Immunol 2005;116:565-70. [PubMed]
- McGarvey LP, Forsythe P, Heaney LG, et al. Bronchoalveolar lavage findings in patients with chronic nonproductive cough. Eur Respir J 1999;13:59-65. [PubMed]
- Boulet LP, Milot J, Boutet M, et al. Airway inflammation in nonasthmatic subjects with chronic cough. Am J Respir Crit Care Med 1994;149:482-9. [PubMed]
- Birring SS, Parker D, Brightling CE, et al. Induced sputum inflammatory mediator concentrations in chronic cough. Am J Respir Crit Care Med 2004;169:15-9. [PubMed]
- Brightling CE, Symon FA, Birring SS, et al. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax 2003;58:528-32. [PubMed]
- Jatakanon A, Lalloo UG, Lim S, et al. Increased neutrophils and cytokines, TNF-alpha and IL-8, in induced sputum of non-asthmatic patients with chronic dry cough. Thorax 1999;54:234-7. [PubMed]
- Xie S, Macedo P, Hew M, et al. Expression of transforming growth factor-beta (TGF-beta) in chronic idiopathic cough. Respir Res 2009;10:40. [PubMed]
- Costello RW, Schofield BH, Kephart GM, et al. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol 1997;273:L93-103. [PubMed]
- Kingham PJ, McLean WG, Sawatzky DA, et al. Adhesion-dependent interactions between eosinophils and cholinergic nerves. Am J Physiol Lung Cell Mol Physiol 2002;282:L1229-38. [PubMed]
- Gu Q, Wiggers ME, Gleich GJ, et al. Sensitization of isolated rat vagal pulmonary sensory neurons by eosinophil-derived cationic proteins. Am J Physiol Lung Cell Mol Physiol 2008;294:L544-52. [PubMed]
- Choudry NB, Fuller RW, Pride NB. Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis 1989;140:137-41. [PubMed]
- Kwong K, Lee LY. Prostaglandin E2 potentiates a TTX-resistant sodium current in rat capsaicin-sensitive vagal pulmonary sensory neurones. J Physiol 2005;564:437-50. [PubMed]
- Niimi A, Nguyen LT, Usmani O, et al. Reduced pH and chloride levels in exhaled breath condensate of patients with chronic cough. Thorax 2004;59:608-12. [PubMed]
- Patterson RN, Johnston BT, Ardill JE, et al. Increased tachykinin levels in induced sputum from asthmatic and cough patients with acid reflux. Thorax 2007;62:491-5. [PubMed]
- Chang AB, Gibson PG, Ardill J, et al. Calcitonin gene-related peptide relates to cough sensitivity in children with chronic cough. Eur Respir J 2007;30:66-72. [PubMed]
- O’Connell F, Springall DR, Moradoghli-Haftvani A, et al. Abnormal intraepithelial airway nerves in persistent unexplained cough? Am J Respir Crit Care Med 1995;152:2068-75. [PubMed]
- Hope-Gill BD, Hilldrup S, Davies C, et al. A study of the cough reflex in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:995-1002. [PubMed]
- Yang XD, Liu Z, Liu HX, et al. Regulatory effect of nerve growth factor on release of substance P in cultured dorsal root ganglion neurons of rat. Neurosci Bull 2007;23:215-20. [PubMed]
- Millqvist E, Ternesten-Hasséus E, Ståhl A, et al. Changes in levels of nerve growth factor in nasal secretions after capsaicin inhalation in patients with airway symptoms from scents and chemicals. Environ Health Perspect 2005;113:849-52. [PubMed]
- O’Connell F, Thomas VE, Studham JM, et al. Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med 1996;90:279-86. [PubMed]
- Dicpinigaitis PV, Bhat R, Rhoton WA, et al. Effect of viral upper respiratory tract infection on the urge-to-cough sensation. Respir Med 2011;105:615-8. [PubMed]
- Eccles R, Lee PC. Cough induced by airway vibration as a model of airway hyperreactivity in patients with acute upper respiratory tract infection. Pulm Pharmacol Ther 2004;17:337-42. [PubMed]
- Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg 2006;135:17-21. [PubMed]
- Jeyakumar A, Brickman TM, Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope 2006;116:2108-12. [PubMed]
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. [PubMed]
- Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol 2005;114:253-7. [PubMed]
- Morgan V, Pickens D, Gautam S, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005;54:601-7. [PubMed]
- Abdi S, Lee DH, Chung JM. The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesth Analg 1998;87:1360-6. [PubMed]
- Governo RJ, Morris PG, Marsden CA, et al. Gabapentin evoked changes in functional activity in nociceptive regions in the brain of the anaesthetized rat: an fMRI study. Br J Pharmacol 2008;153:1558-67. [PubMed]


