
Long-term oncological outcome after thoracoscopic lobectomy for non-small cell lung cancer patients
Introduction
Lobectomy with systematic lymph node dissection is considered the gold standard treatment for patients with early-stage non-small cell lung cancer (NSCLC). During the previous two decades, thoracoscopic surgery (TS), also known as video-assisted thoracoscopic surgery (VATS), has become commonly used as a less invasive procedure than conventional thoracotomy (TH) worldwide. In Japan, in 2013, of the 37,008 operations for primary lung cancers, 18,925 were TS lobectomies (1). They comprised 69% of all lobectomies, and this proportion is gradually increasing. The recent increase in the use of TS is due to the results of several reported data of retrospective and prospective studies that show comparable treatment outcomes and less-invasiveness of TS as compared to those of TH. Although some differences exist in the manner or indication between individual institutes or thoracic surgeons, many surgeons believe that TS is a superior approach than TH for standard surgery in lung cancer.
The lower invasiveness of TS, as compared to that of TH, is very challenging to prove statistically because the evaluation items for measuring invasiveness, such as postoperative pain and quality of life, depend on patient subjectivity. However, in 2016, the results of a well-designed randomized controlled trial (RCT) to compare the invasiveness of TS and anterolateral TH for early-stage NSCLC were reported in Denmark (2). In the report, the authors showed TS is associated with less postoperative pain and better quality of life than is anterolateral TH for the first year after surgery.
However, there are few prospective RCTs to compare patient survival between TS and TH in patients with NSCLC (3). Moreover, these studies were all reported during the late 1990s and early 2000s, when TS was just beginning to be used, therefore, these data may not be applicable to the current clinical practice because of skill progressions in the TS procedures and changes in the characteristics of NSCLC patients. Most of the recently reported data are from retrospective studies (4-8). We should know that the quality of these studies varies, and they do not provide sufficient evidence as compared with that provided by RCTs. To interpret these data, even though they may be large-scale data from a national database or multicenter studies, we must consider the possibility of selection bias in the patients and differences in the TS procedures among the institutes. Although we should compare the survival benefit between TS and TH in a multicenter RCT, it would be challenging to perform this comparison in Japan. One of the reasons is that TS is already popular as a treatment procedure for early-stage NSCLC. Therefore, majority of Japanese thoracic surgeons would feel not be comfortable performing TH for patients with early diseases, even for clinical trials. Second, several kinds of TS procedures coexist owing to the lack of a clear definition of TS or VATS, and each procedure is embedded in each institute in Japan. It is difficult to deal with all the procedures under the TS or VATS group together, with a varying length and number of wounds.
This study aimed to conduct a retrospective comparison of the long-term oncological outcomes in NSCLC patients who undergo TS and TH, in a single cancer specialized institute in Japan.
Methods
Patients
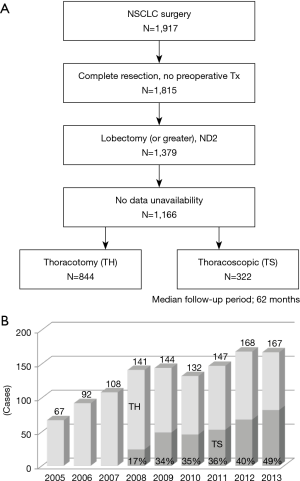
Study patient flow diagram is shown in Figure 1A. Total 1,917 consecutive NSCLC patients underwent surgery at our institution between January 2005 and December 2013. Of them, 1,815 underwent complete surgical resection without any preoperative anticancer treatment. Excluding the cases with any kind of limited surgery, 1,379 patients underwent lobectomy or more extensive resection with systematic ipsilateral hilar and mediastinal lymph node dissection. Further, 1,166 patients whose data were available were finally enrolled in this retrospective study. We reviewed the medical records of all the patients for the following clinicopathological factors: age, sex, smoking index (pack-year), comorbidity, respiratory function (%FVC and FEV1.0%), operative procedure, clinical and pathological T and N status, histological type, radiological findings of the primary lesion, operative time, blood loss in operation, postoperative complications, and drainage period. Histological classification of the resected specimens was determined according to the World Health Organization International Histological Classification of Tumors (9). Clinical and pathological stage were determined according to the 7th UICC TNM Classification for Lung and Pleural Tumors (10). Radiological findings were determined based on the findings of high-resolution computed tomography (HRCT) images of the primary lesions, and the patients were divided into two groups, ground glass opacity (GGO) dominant tumor or solid dominant tumor. Postoperative complications were assessed according to the Clavien-Dindo classification (11). Data collection and analysis were approved by the Institutional Review Board (No. 2018-1089). The need to obtain written informed consent from each patient was waived because of the retrospective nature of the study and anonymity of the subjects.
Surgical procedures of TS and TH
The surgical procedure of TS was performed via a 2.5 cm utility incision at the posterior axillary line at the 5th intercostal space using endoscopic instruments and traditional instruments for sharp dissection. The latissimus dorsi muscle was left intact, the serratus anterior muscle was split and the intercostal muscle minimally divided about 4 cm to remove the resected specimens. A 15-mm incision was created at the anterior axillary line at the 4th intercostal space for the second assistant. Two 7-mm ports were placed at the center of the 3rd intercostal space for the camera and posteriorly at the 3rd intercostal space for the operator’s left hand. Two silicone rubber instruments (Lap Protector™ Minimini; Hakko Co, Ltd., Tokyo, Japan) were applied to maintain the two wounds open. All the procedures were strictly monitor-based and the role of each port was fixed. The TH operations were performed in a conventional manner via a posterolateral incision, about 18 cm, with rib dividing. The latissimus dorsi muscle, serratus anterior muscle, and intercostal muscle were well divided.
Statistical analyses
Either the Chi-square test or Fisher’s exact test was used to analyze the correlations between the operative approaches and the categorical variables of several clinicopathological factors. Student’s t-test was applied to analyze continuous variables, such as age, operative time and blood loss, and postoperative drainage period. Survival curves were plotted using the Kaplan-Meier method, and the statistical significance of differences among the subgroups was determined using the log-rank test. Overall survival (OS) was measured from the date of surgery to the date of death due to any cause. The last follow-up observation was censored when the patient was alive or lost to follow-up. Cancer-specific survival (CSS) was measured from the date of surgery to the date of death due to lung cancer. The last follow-up observation was censored when the patient was alive, lost to follow-up or death due to other causes. All P values were two-sided, and P<0.05 was considered to indicate a statistical significance. Propensity scores were estimated using a logistic regression model including the following variables: age, sex, smoking index, FEV1.0%, clinical T and N factors, radiological findings and histology of the primary lesions. Thereafter, the nearest neighbor 1:1 matching method was used without replacement. We used the statistical analysis software (Dr. SPSS II for Windows, Standard Version 11.0, SPSS Inc., Chicago, IL, USA) for all analyses.
Results
Comparisons of the TH and TS groups of the entire cohort
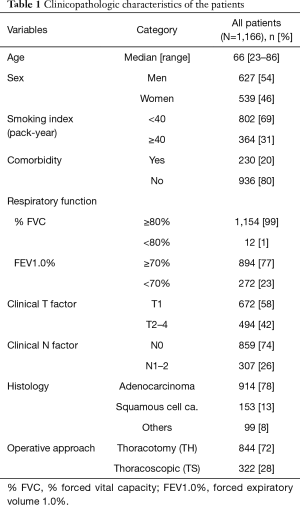
The clinicopathologic characteristics of the 1,166 enrolled patients are shown in Table 1. Non-smokers or light smokers (smoking index <40 pack-year), the patients without any comorbidity and those with normal respiratory function comprised a majority of the cohort. Clinical T1, N0, and adenocarcinoma histology were the major disease states. Of the 1,166 patients, 844 were underwent surgery via TH and 322 via TS. The annual change in the percentages of TS during the study period is shown in Figure 1B.
Full table
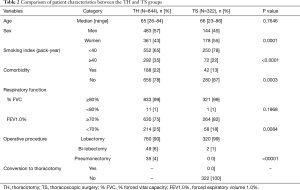
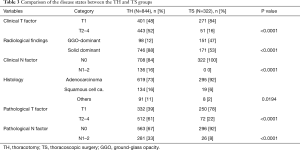
Comparisons of the patient characteristics and disease states between the TH and TS groups are shown in Tables 2 and 3. The TS group included significantly more women, non-smokers or light smokers, patients without any comorbidity and those with normal FEV1.0% than the TH group. The rate of lobectomy in all the procedures was high in the TS group. These results are summarized as follows: the patients in good conditions were favored for TS in clinical practice. There were no conversion cases from via TS to TH during the study period. In the TS group, the disease states were significantly less aggressive with low clinical and pathological T and N status than in the TH group. Moreover, almost 50% of the tumors in the TS group presented GGO-dominant shadows by TSCT.
Full table
Full table
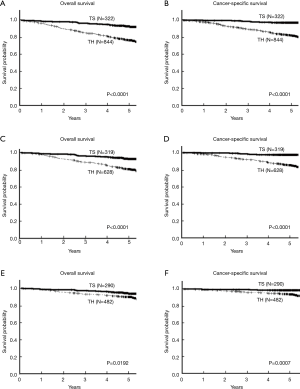
The survival curves of the two groups are shown in Figure 2. The TS group had a much better 5-year OS rate of 92.6% than the TH group (76.7%), and the log-rank test showed a statistically significant difference (P<0.0001; Figure 2A). Similar results were obtained for CSS, a more suitable indicator of oncological outcomes than OS. The TS group had an excellent oncological outcome that was significantly better than that of the TH group (5-year CSS rate 97.4% in the TS group vs. 82.2% in the TH group, P<0.0001; Figure 2B). A comparison of the patients with clinical stage I disease, shown in Figure 2C,D, showed significantly better 5-year OS rate (92.8% vs. 81.0%, P<0.0001) and 5-year CSS rate (97.7% vs. 85.5%, P<0.0001) were better in the TS group. In patients with pathological stage I disease, although the differences were lower, the parameters were significantly better in the TS group (5-year OS rates; 93.9% vs. 90.0%, P=0.0192; Figure 2E and 5-year CSS rates; 98.9% vs. 94.0%, P=0.0007; Figure 2F). These differences in patient outcomes are attributable to unavoidable selection bias.
Comparisons of the TH and TS groups of the matched cohort
We performed propensity score matching to minimize the differences in background patient characteristics and tumor states between the TH and TS groups. We extracted 190 patients each from the two groups. We confirmed the absence of significant differences in the patient characteristics between the two matched groups, as shown in Table 4. With respect of the disease states, the factors that can be assessed preoperatively, such as the clinical T and N status, radiological findings and tumor histology were comparable (Table 5).

Full table

Full table
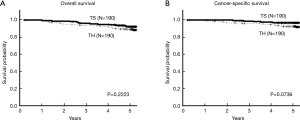
The survival curves of the two matched groups are shown in Figure 3. There were no statistical differences in the OS and CSS rates between the two groups (P=0.2223 and P=0.0736), indicating the achievement of adequate balance.
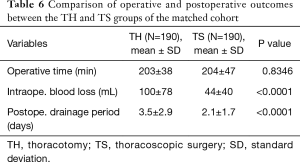
For a balanced cohort, we compared the operative and postoperative outcomes between the TH and TS groups (Tables 6 and 7). Although the operative time was similar, blood loss was significantly less and the duration of postoperative drainage was significantly shorter in the TS group than in the TH groups. There were no significant differences in the frequency of postoperative complications (Clavien-Dindo classification Grades II-V) between the two groups. The common Grade II and III complications of the TS group were supraventricular arrhythmia, requiring administration of anti-arrhythmic drugs and pulmonary fistula that required pleurodesis.
Full table

Full table
Discussion
This single-institute, retrospective study compared the long-term oncological outcomes in patients with NSCLC who underwent TS and TH. The present findings accurately demonstrate our clinical practice. In our institute, TS lobectomy was introduced in 2008, shown in Figure 1B. At that time, the operative indication for TS lobectomy in NSCLC was limited in patients with GGO-dominant lesions, up to 3 cm in diameter, and only 17% of lobectomies were performed via TS. However, thereafter, the operative indication of TS lobectomy was gradually extended, and the rate gradually increased with advances in the procedure technology. We expanded the indication of TS lobectomy to the GGO-dominant lesions up to 5 cm in diameter and solid dominant lesions up to 3 cm in size. Then, in 2013, 49% of lobectomies for NSCLC were performed via TS. The survival difference between the TS and TH groups shown in Figure 2 was a result of the selection bias in our clinical practice. Patients with less aggressive lesions tended to be subjected to TS during the study period, although the rate of TS gradually increased. The survival rates were equal in the two matched cohorts, as shown in Figure 3. These results show an adequate balance after matching. According to the 8th TNM classification, GGO component, reflecting pathological non-invasive part, means less-aggressiveness of the tumor (12). The data in Table 3 shows that TS tend to be performed for patients with GGO-dominant lesions. Thus, to avoid selection bias, it is crucial to include not only T and N factors but also the radiological findings of the tumor into a logistic regression model for propensity score estimating.
With respect to the curability of lung cancer after TS lobectomy, majority of the recently reported data were from retrospective studies. Yang et al. reported on the long-term after TS or TH lobectomy based on the National Cancer Data Base of U.S. (6). About three thousand patients with stage I NSCLC were matched with propensity score from >7,000 patients; the 5-year OS rates of the two groups were similar. Although these results were comparable to our findings, the reported 5-year OS rates, 65–66%, were relatively low for stage I disease. CSS rates may give more accurate results; however, it would be challenging to access in detail from the nationwide database. Flores et al. reported the results of a retrospective study that compared the curability between TS and TH for stage I NSCLC (4). Total 398 patients who underwent TS lobectomy and 343 patients who underwent TH demonstrated similar 5-year OS rates, 79% and 75%, respectively. These data were from a single-institute, smaller-scale trial; however, the study was conducted in a very specialized institute, and the data were well analyzed.
There are few RCT reports, including the one by Sugi et al. that compares patient survival between TS and TH (3). Certainly, these studies have reported important data with higher levels of evidence than those in retrospective studies; however, the sample size of these studies does not permit statistically valid conclusions.
Our results showed much better oncological outcomes than previous reports, with a 5-year OS rate of 92.6% and CSS rate of 97.4% after TS lobectomy. The patient characteristics of the patients in our institute, shown in Table 3, are one of the reasons for these results. In the urban areas of Japan, GGO-dominant lesions are increasingly detected in health screening with the increasing popularity of CT and PET-CT in recent years. The patients with GGO-dominant lesions have an extremely favorable prognosis after complete resection. For some early diseases, we also perform TS segmentectomy. Peripherally located GGO-dominant lesions <2 cm in size are our intentional indication for segmentectomy. We also reported equivalent outcomes, with a 5-year OS rate of 93.4% in the 191 patients who underwent TS segmentectomy in 2018 (13).
The study period also influences patient outcomes. Diverse and effective treatments after cancer recurrence, including the use of molecular-targeted agents and immune checkpoint inhibitors are available.
With respect to the less-invasiveness of TS, to our knowledge, only four prospective studies have been reported in the English literature (2,14-16). Kirby et al. reported the results of an RCT on 61 patients with stage I NSCLC in 1995 (14). There were no significant differences in the operating time, intraoperative blood loss, duration of chest tube drainage, or length of hospital stay between the TS and TH groups. However, TH showed significantly longer air leakage. Craig et al. conducted a RCT in 2001 and showed that TS was associated with reduced peri-operative changes in the acute phase response (15). They showed that TS was associated with lower CRP and IL-6 levels after 24–120 hours after surgery than TH. Long et al. reported short-term outcomes of RCT to compare TS and TH lobectomy in 2018 (16). They randomly assigned 481 early-stage NSCLC patients (1:1 ratio) at five tertiary hospitals in China. The median operation time and intraoperative blood loss was significantly less with TS than with TH lobectomy. The length of hospitalization and morbidities were similar in the two groups. An uniquely designed RCT conducted in Denmark in 2016 enrolled 206 patients with stage I NSCLC and randomly assigned them to the TS or anterolateral TH groups (1:1 ratio) (2). After the operation, an identical surgical dressing was applied to all the patients to mask the type of the surgical procedure from the surgeon and the patient until discharge. The authors showed that the TS group had a lower incidence of significant pain in the first 24 hours after surgery and of relevant pain episodes during the 52-week follow-up period, with a better postoperative quality of life. With respect to the latter two recent trials, long-term patient outcomes results are expected.
Our results showed that the operative blood loss and the length of drainage period were evidently less and shorter in the TS group. These results proved the lower invasiveness of TS. Because of the retrospective nature of this study, it was difficult to prove less postoperative pain and better QOL after TS than TH. However, length of skin incision is evidently shorter and range of divided muscles is narrower after TS, which contribute to a high satisfaction level of patients. About operative safety of TS, we did not have an experience of conversion to TH in this study period, shown in Table 2. Although this is an extremely good result, we experience one conversion case of segmentectomy in the same period.
Recently, several new efforts are being made for the development of less-invasive approaches, such as uniportal-VATS (17-19) and robotic surgery (20,21). It may be challenging to launch RCTs by the moment to compare each new approach with the conventional method. The most important essence of surgery for lung cancer must be the oncological curability. It should be assessed as fairly as possible for every less-invasive approach, regardless of the study design (prospective or retrospective).
Conclusions
We reported the long-term oncological outcomes in patients with early-stage NSCLC after TS lobectomy. Although this is a single institutional, retrospective study, we successfully avoided selection bias in the patients and showed comparable treatment outcomes with lower invasiveness of TS as compared to that of TH.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Data collection and analysis were approved by the Institutional Review Board (No. 2018-1089). The need to obtain written informed consent from each patient was waived because of the retrospective nature of the study and anonymity of the subjects.
References
- Masuda M, Kuwano H, Okumura M, et al. Thoracic and cardiovascular surgery in Japan during 2013: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2015;63:670-701. [Crossref] [PubMed]
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000;24:27-30. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Yang CJ, Kumar A, Klapper JA, et al. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann Surg 2019;269:163-71. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of Lung, Pleura, Thymus and Heart. 4th ed. Lyon: IASLC Press; 2015.
- Sobin LH, Gospodrowicz MK, Wittekind CH. TNM Classification of Malignant Tumours, 7th ed. New York, NY: Wiley-Blackwell; 2009.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Mun M, Nakao M, Matsuura Y, et al. Thoracoscopic segmentectomy for small-sized peripheral lung cancer. J Thorac Dis 2018;10:3738-44. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001. [Crossref] [PubMed]
- Craig SR, Leaver HA, Yap PL, et al. Acute phase responses following minimal access and conventional thoracic surgery. Eur J Cardiothorac Surg 2001;20:455-63. [Crossref] [PubMed]
- Long H, Tan Q, Luo Q, et al. Thoracoscopic Surgery Versus Thoracotomy for Lung Cancer: Short-Term Outcomes of a Randomized Trial. Ann Thorac Surg 2018;105:386-92. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Dai F, Meng S, Mei L, et al. Single-port video-assisted thoracic surgery in the treatment of non-small cell lung cancer: a propensity-matched comparative analysis. J Thorac Dis 2016;8:2872-8. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]


