Efficacy and safety of crizotinib in patients with ROS1 rearranged non-small cell lung cancer: a retrospective analysis
Introduction
Protein kinase activation induced by somatic mutation or chromosomal alteration is one of the mechanisms of tumorigenesis and has led to targeted therapies with specific inhibitor drugs (1). For patients with non-small cell lung carcinoma (NSCLC) harboring driver gene mutations and rearrangements, the use of small-molecule tyrosine kinase inhibitors (TKIs) has been a standard therapy (2,3). Oncogenic c-ros oncogene 1 (ROS1) is one of the rearrangements found in NSCLC. The ROS1 gene-fuses to several partner genes, and the activated ROS1 fusion kinases drive cellular transformation (4-6). ROS1 fusion is generally known to be present in approximately 1–3% of NSCLC cases, and an effective TKI is available (7-9).
For ROS1 rearranged NSCLC (ROS1-NSCLC), crizotinib, a small-molecule TKI, has been used. Crizotinib was initially approved for ALK-rearranged NSCLC. In 2012, the possibility that crizotinib might be exquisitely effective against ROS1-NSCLC in vitro was reported (7). In addition, several reports showed its efficacy in patients with ROS1-NSCLC. Thereafter, two prospective cohort studies were conducted: PROFILE 1001 and OO12-01. PROFILE 1001 was a phase 1 expansion study evaluating the efficacy and safety of crizotinib in 50 patients with ROS1-NSCLC (10). The objective response rate (ORR) was 72.0% [95% confidence interval (CI), 58.0 to 84.0], and the median progression-free survival (PFS) was 19.2 months (95% CI, 14.4 to not reached). OO12-01 was a large phase 2 study that enrolled 127 patients with ROS1-NSCLC. It demonstrated a clinically meaningful benefit and durable responses with crizotinib in East-Asian patients (11). The ORR was 71.7% (95% CI, 63.0 to 79.3), and the median PFS was 15.9 months (95% CI, 12.9 to 24.0). Based on these two studies, crizotinib was approved for the treatment of ROS1-NSCLC in Japan in May 2017. However, because of the small number of patients with ROS1-NSCLC, the efficacy and safety of crizotinib in clinical practice has been poorly documented in Japan.
In this report, we retrospectively reviewed the clinical characteristics of patients with ROS1-NSCLC and sought to assess the efficacy and safety of crizotinib in Japanese patients in actual clinical practice.
Methods
Subjects
Between December 2014 and May 2018, patients with a definite diagnosis of advanced/relapsed ROS1-NSCLC were selected from consecutive NSCLC cases treated at the National Cancer Center Hospital. The diagnosis of ROS1-NSCLC was primarily based upon reverse transcription polymerase chain reaction (RT-PCR), fluorescence in situ hybridization (FISH), or next generation sequencing (NGS). We reviewed the patients’ medical records and collected the following information: patient characteristics, histology, treatment history, and methods of ROS1 detection. Especially, we assessed the efficacy of the most commonly used previous treatment regimens.
Treatment and assessment
We extracted patients with ROS1-NSCLC who had been treated with crizotinib in actual clinical practice. We excluded patients in whom the efficacy of the treatment could not be evaluated or who had participated in clinical trials for ROS1-NSCLC. For the efficacy analysis, we included patients who had at least one measurable lesion and had undergone a computed tomography evaluation 6 to 8 weeks after the start of crizotinib therapy. We evaluated the efficacy of the treatment based on the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). PFS was defined as the time from the beginning of treatment until disease progression or death, and overall survival (OS) was measured from the initiation of treatment until the date of death. PFS was censored as of the last date on which the patient was known to be progression-free, and OS was censored as of the date of the last follow-up. If a patient changed to another treatment because of toxicity, we handled them as censored cases as of the beginning of the next treatment. In the safety analysis, we assessed all the patients with ROS1-NSCLC who were treated with crizotinib in clinical practice. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, ver. 4.03. The survival rates were estimated using the Kaplan-Meier method. All the statistical analyses were performed using JMP version 14.0 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
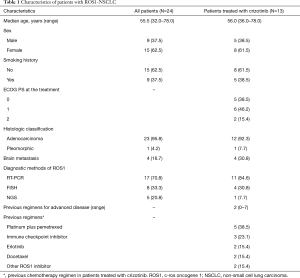
During the study period, 24 patients (1.9%) were diagnosed as having ROS1-NSCLC. The baseline characteristics of the patients are shown in Table 1. The ROS1 rearrangement status was assessed using RT-PCR (n=17), FISH (n=8), or NGS (n=5). Among the 24 patients who were diagnosed as having ROS1-NSCLC, the 13 patients who were treated with crizotinib in actual clinical practice (female, n=8; male, n=5) had a median age of 56 years (range, 36–78 years). Twelve patients had adenocarcinoma, and 8 were never-smokers. The median number of prior chemotherapy treatments before crizotinib was 2 (range, 0–7). Two and three patients received crizotinib as first and second line, respectively. Among the 11 patients who did not receive crizotinib in clinical practice, 5 received crizotinib as part of investigator sponsored trials for ROS1-NSCLC. We identified the following fusion partners of ROS1: CD74 molecule gene (CD74; n=3), syndecan 4 gene (SDC4; n=1), and solute carrier family 34 member 2 (SLC34A2; n=1).
Full table
Treatment efficacy and toxicity
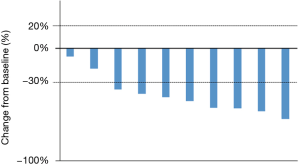
Patients received the standard crizotinib dose of 250 mg twice a day until one of the following events occurred; disease progression, clinical deterioration, or unacceptable toxicity. When AEs were related to crizotinib, the dose of crizotinib was modified depending on the grade of the adverse events. Among 13 patients, three patients were excluded because they were not fully performed imaging evaluations. In the 10 evaluable patients, the median follow-up time was 35.5 months (95% CI, 8.9 to 44.6 months), the median PFS was 10.0 months (95% CI, 5.1 to 27.0 months), and the OS was 28.7 months (95% CI, 6.7 to not reached) (Figure 1). A waterfall plot of the patients in whom the response could be evaluated is shown in Figure 2. The best overall responses were a partial response (PR) in 8 patients and stable disease (SD) in 2 patients. The ORR was 80.0% (95% CI, 49.0 to 94.3). In terms of the efficacy of the previous treatments that had been performed in 18 patients, 11 patients received pemetrexed/platinum and 7 patients received ICIs. The ORRs for treatment with pemetrexed/platinum and immune checkpoint inhibitors (ICIs) were 45.5% (95% CI, 21.3 to 72.0) and 14.3% (95% CI, 2.6 to 51.3), respectively. A swimmer’s plot of the duration of the previous chemotherapies in the 13 patients who received crizotinib in clinical practice is shown in Figure 3.

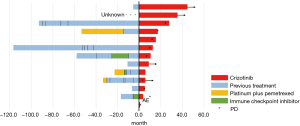
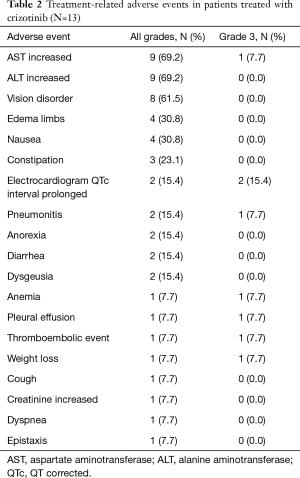
Table 2 shows the details of the adverse events in the 13 patients who received at least one dose of crizotinib in clinical practice. The most frequent adverse events were aspartate aminotransferase (AST) increased and alanine aminotransferase (ALT) increased (69.2%). Overall, the number of grade 3 adverse events was 8: electrocardiogram QT corrected (QTc) interval prolonged (n=2), anemia, AST increased, weight loss, pleural effusion, pneumonitis and thromboembolic event (all n=1). Regarding the patient with grade 3 pneumonitis, the physician suspected interstitial lung disease (ILD) related to crizotinib treatment and discontinued the treatment. In contrast, the other patients with grade 3 adverse events continued crizotinib treatment after a treatment interruption or dose reduction. No grade 4 or 5 adverse events related to crizotinib were reported.
Full table
Discussion
Since crizotinib was first approved for the treatment of ROS1-NSCLC in Japan in May 2017, we have only been able to treat a few ROS1-NSCLC patients with crizotinib in clinical practice in Japan. In addition, ROS1-NSCLC itself is a rare lung cancer, and an even smaller number of patients are treated with crizotinib at individual hospitals in Japan (12). For this reason, we reviewed the efficacy and safety of crizotinib in clinical practice at our hospital.
In addition to PROFILE 1001 and OO12-01, several studies have reported the efficacy and safety of crizotinib for the treatment of ROS1-NSCLC. The EUROS1 cohort was assembled from six European countries (13). The investigators retrospectively identified 32 patients who had received crizotinib for the treatment of ROS1-NSCLC. The median PFS was 9.1 months, the response rate was 80%, and no unexpected adverse effects were observed in their study. Park et al. reported the characteristics and outcomes of ROS1-NSCLC patients in clinical practice in Korea (14). Within their cohort, 15 patients received crizotinib. The median PFS was 13.1 months, and the response rate was 73.3%. Noronha et al. reported that crizotinib resulted in durable disease control and prolonged PFS in 5 ROS1-NSCLC patients of India (15). In our study, the median PFS was 10.0 months, the OS was 28.7 months, and the response rate was 80%. Our results are similar to those of previous studies and thus support the efficacy and safety of crizotinib for clinical use in Japan. Some previous studies have indicated that pemetrexed-based therapies and ICIs are effective in patients with ROS1-NSCLC (16-19). In our study, 11 patients received pemetrexed-based therapies and 7 patients received ICIs. The ORR of pemetrexed/platinum and ICIs were 45.5% (95% CI, 21.3 to 72.0) and 14.3% (95% CI, 2.6 to 51.3), respectively. The efficacy was relatively the same as that of previous reports. Although our results were for a relatively small sample size, pemetrexed-based therapies and ICIs might be effective in patients with ROS1-NSCLC in clinical settings.
Similar to previous clinical trials, we were able to continue to treat patients with ROS1-NSCLC using crizotinib safely. A QTc interval prolonged which 2 patients experienced, was the most common grade 3 adverse event (15.4%) in our study. We previously reported that patients with an ATP-binding cassette sub-family B member 1 (ABCB1) genotype and a lower body weight were more likely to develop severe adverse events among Japanese NSCLC patients harboring ALK fusion gene treated with crizotinib (20). We were aware of the potential cardiotoxicity of crizotinib and were able to avoid fatal adverse events through frequent electrocardiogram examinations. After stopping crizotinib treatment and confirming recovery, we were able to continue treatment after a dose reduction of crizotinib before the AE became lethal. We suggest that physicians should be cautious of QTc interval prolonged results in patients receiving crizotinib.
Recently, several studies discussing the mechanism of resistance to crizotinib in ROS1-NSCLC have been published. Most patients with ROS1-NSCLC invariably acquire resistance to crizotinib despite its initial efficacy. Gainor et al. reported that they identified 16 patients who underwent a total of 17 repeat biopsies following progression while receiving crizotinib, and they identified ROS1 resistance mutations in 53% of the specimens (21). In their study, ROS1 mutations included G2032R (41%), D2033N (6%), and S1986F (6%). Other ROS1 resistance mutations have been described in some studies (22-25). Moreover, activations of KIT, KRAS and EGFR have been identified as mechanisms of resistance to crizotinib in ROS1-NSCLC (26-28). These studies are expected to lead to a large step forward in the development of new drugs for the treatment of ROS1-NSCLC with acquired crizotinib resistance. Recently, the efficacy of a new generation of ROS1 inhibitors, including lorlatinib and entrectinib, has been shown in early clinical trials for ROS1-NSCLC (29-31).This study had some limitations. First, the study was performed retrospectively at a single center in Japan. In this regard, it is impossible to compare our results with other global results completely. Second, the numbers and types of previous regimens differed among the patients with ROS1-NSCLC. These previous regimens might have influenced the results for the efficacy and toxicity of crizotinib.
Conclusions
Our results demonstrated that the administration of crizotinib to patients with ROS1-NSCLC was effective and safe in clinical practice in Japan.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Fujiwara reports grants from Abbvie, grants and personal fees from Astra Zeneca, grants and personal fees from BMS, grants from Chugai, grants from Daiichi-Sankyo, grants from Eisai, grants from Eli Lilly, grants from Incyte, grants from Merck Serono, grants and personal fees from MSD, grants and personal fees from Novartis, personal fees from ONO, personal fees from Sysmex, personal fees from Taiho, outside the submitted work. Dr. Matsumoto reports grants from Hitachi, grants from Hitachi High-Technologies, grants from Boston Scientific, personal fees from Olympus, personal fees from Cook Medical, personal fees from AstraZeneca, outside the submitted work. Dr. Murakami reports grants from Takeda Pharmaceutical, personal fees from Astrazeneca, personal fees from Chugai Pharmaceutical, personal fees from Boehringer Ingelheim, personal fees from Taiho Pharmaceutical, personal fees from Ono Pharmaceutical, outside the submitted work. Dr. Goto reports grants and personal fees from Eli Lilly, personal fees from Chugai, grants and personal fees from Taiho Pharmaceutical, personal fees from Boehringer Ingelheim, personal fees from Pfizer, personal fees from Novartis, personal fees from Glaxo Smith Kline, personal fees from AstraZeneca, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Bristol Myers Squibb, personal fees from Shionogi Pharma, personal fees from Merck Sharp and Dohme, grants from Abbvie, from null, outside the submitted work. Dr. Kanda reports grants and personal fees from AstraZeneca, grants and personal fees from Ono Pharmaceutical, grants from AbbVie, personal fees from Bristol-Myers Squibb, personal fees from Chugai, outside the submitted work. Dr. Horinouchi reports grants and non-financial support from Ono, during the conduct of the study; grants and personal fees from BMS, grants and personal fees from Novartis, grants from Astellas, grants and personal fees from Taiho, grants and personal fees from Chugai, personal fees from Lilly, grants and personal fees from Astra Zeneca, grants and personal fees from MSD, grants from Merck Serono, grants from Genomic Health, outside the submitted work. Dr. Yamamoto reports grants from Chugai, grants from Taiho, grants from Eisai, grants from Lilly, grants from Quintiles, grants from Astellas, grants from BMS, grants from Novartis, grants from Daiichi-Sankyo, grants from Pfizer, grants from Boehringer Ingelheim, grants from Kyowa-Hakko Kirin, grants from Bayer, grants from ONO PHARMACEUTICAL CO., LTD, grants from Takeda, personal fees from ONO PHARMACEUTICAL CO., LTD, personal fees from Chugai, personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Lilly, personal fees from BMS, personal fees from Eisai, personal fees from Otsuka, personal fees from Takeda, personal fees from Boehringer Ingelheim, personal fees from Cimic, grants from Janssen Pharma, grants from MSD, grants from Merck, outside the submitted work. Dr. Ohe reports grants and personal fees from Pfizer, during the conduct of the study; grants and personal fees from AstraZeneca, grants and personal fees from Chugai, grants and personal fees from ONO, personal fees from Celltrion, personal fees from Amgen, grants and personal fees from Kyorin, grants and personal fees from Takeda, grants and personal fees from Lilly, grants and personal fees from BMS, grants and personal fees from MSD, personal fees from Boehringer Ingelheim, grants and personal fees from Taiho, grants from Kissei, personal fees from Novartis, grants from Dainippon-Sumitomo, grants from Ignyta, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of the National Cancer Center Hospital (No. 2015-355). Due to the retrospective nature of this study, informed consent was not obtained from each patient.
References
- Puig de la Bellacasa R, Karachaliou N, Estrada-Tejedor R, et al. ALK and ROS1 as a joint target for the treatment of lung cancer: a review. Transl Lung Cancer Res 2013;2:72-86. [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378-81. [Crossref] [PubMed]
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [Crossref] [PubMed]
- Kohno T, Nakaoku T, Tsuta K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res 2015;4:156-64. [PubMed]
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70. [Crossref] [PubMed]
- Birchmeier C, Sharma S, Wigler M. Expression and rearrangement of the ROS1 gene in human glioblastoma cells. Proc Natl Acad Sci U S A 1987;84:9270-4. [Crossref] [PubMed]
- Gu TL, Deng X, Huang F, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One 2011;6:e15640. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Wu YL, Yang JC, Kim DW, et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:1405-11. [Crossref] [PubMed]
- Saito M, Shiraishi K, Kunitoh H, et al. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci 2016;107:713-20. [Crossref] [PubMed]
- Mazieres J, Zalcman G, Crino L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol 2015;33:992-9. [Crossref] [PubMed]
- Park S, Ahn BC, Lim SW, et al. Characteristics and Outcome of ROS1-Positive Non-Small Cell Lung Cancer Patients in Routine Clinical Practice. J Thorac Oncol 2018;13:1373-82. [Crossref] [PubMed]
- Noronha V, Chandrakanth MV, Joshi AP, et al. ROS1 rearranged nonsmall cell lung cancer and crizotinib: An Indian experience. Indian J Cancer 2017;54:436-8. [Crossref] [PubMed]
- Chen YF, Hsieh MS, Wu SG, et al. Efficacy of Pemetrexed-Based Chemotherapy in Patients with ROS1 Fusion-Positive Lung Adenocarcinoma Compared with in Patients Harboring Other Driver Mutations in East Asian Populations. J Thorac Oncol 2016;11:1140-52. [Crossref] [PubMed]
- Zhang L, Jiang T, Zhao C, et al. Efficacy of crizotinib and pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 rearrangement. Oncotarget 2016;7:75145-54. [PubMed]
- Song Z, Su H, Zhang Y. Patients with ROS1 rearrangement-positive non-small-cell lung cancer benefit from pemetrexed-based chemotherapy. Cancer Med 2016;5:2688-93. [Crossref] [PubMed]
- Remon J, Hendriks LE, Cabrera C, et al. Immunotherapy for oncogenic-driven advanced non-small cell lung cancers: Is the time ripe for a change? Cancer Treat Rev 2018;71:47-58. [Crossref] [PubMed]
- Fujiwara Y, Hamada A, Mizugaki H, et al. Pharmacokinetic profiles of significant adverse events with crizotinib in Japanese patients with ABCB1 polymorphism. Cancer Sci 2016;107:1117-23. [Crossref] [PubMed]
- Gainor JF, Tseng D, Yoda S, et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol 2017. doi: 10.1200/PO.17.00063. [Crossref]
- Song A, Kim TM, Kim DW, et al. Molecular Changes Associated with Acquired Resistance to Crizotinib in ROS1-Rearranged Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:2379-87. [Crossref] [PubMed]
- Facchinetti F, Loriot Y, Kuo MS, et al. Crizotinib-Resistant ROS1 Mutations Reveal a Predictive Kinase Inhibitor Sensitivity Model for ROS1- and ALK-Rearranged Lung Cancers. Clin Cancer Res 2016;22:5983-91. [Crossref] [PubMed]
- Watanabe J, Furuya N, Fujiwara Y. Appearance of a BRAF Mutation Conferring Resistance to Crizotinib in Non-Small Cell Lung Cancer Harboring Oncogenic ROS1 Fusion. J Thorac Oncol 2018;13:e66-9. [Crossref] [PubMed]
- McCoach CE, Le AT, Gowan K, et al. Resistance Mechanisms to Targeted Therapies in ROS1(+) and ALK(+) Non-small Cell Lung Cancer. Clin Cancer Res 2018;24:3334-47. [Crossref] [PubMed]
- Dziadziuszko R, Le AT, Wrona A, et al. An Activating KIT Mutation Induces Crizotinib Resistance in ROS1-Positive Lung Cancer. J Thorac Oncol 2016;11:1273-81. [Crossref] [PubMed]
- Cargnelutti M, Corso S, Pergolizzi M, et al. Activation of RAS family members confers resistance to ROS1 targeting drugs. Oncotarget 2015;6:5182-94. [Crossref] [PubMed]
- Davies KD, Mahale S, Astling DP, et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PLoS One 2013;8:e82236. [Crossref] [PubMed]
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590-9. [Crossref] [PubMed]
- Liu D, Offin M, Harnicar S, et al. Entrectinib: an orally available, selective tyrosine kinase inhibitor for the treatment of NTRK, ROS1, and ALK fusion-positive solid tumors. Ther Clin Risk Manag 2018;14:1247-52. [Crossref] [PubMed]
- Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov 2018;8:1227-36. [Crossref] [PubMed]