
Magnolol inhibits growth and induces apoptosis in esophagus cancer KYSE-150 cell lines via the MAP kinase pathway
Introduction
Esophagus cancer is an extremely aggressive malignant tumor with a high clinical incidence. It ranks ninth in terms of incidence and sixth for mortality worldwide (1-3). It affects more than 400,000 people worldwide every year and the incidence rates are rapidly increasing, especially in China (4). Pickled vegetables, tobacco and alcohol are the most common carcinogenic environmental factors that have contributed to the increased incidence of esophageal cancers (5). The morbidity of patients with esophageal cancer in China accounts for over 40% of patients worldwide. It has become a severe threat to people's health and a heavy financial burden to society in general (6). The therapeutic strategy for patients with early-stage esophageal cancer is surgery as the first choice. It can play a role in improving the patient’s prognosis. However, the 5-year survival rate for patients with esophageal cancer is only around 20% (7). Most patients are at the advanced stage of the disease when they are diagnosed. Different therapeutic interventions are available, such as chemo- and radiation therapy. However, these treatment strategies are often inadequate or associated with severe side effects. The alternate treatment options that have gained prominence are phytochemicals, i.e., natural compounds that have fewer side effects, higher specificity and cost-effectiveness.
Medicinal herbs have been used to treat a variety of diseases and have a long history of demonstrated therapeutic benefit. In recent years, there has been a worldwide trend in research of natural compounds present in herbs, vegetables and fruits for their anti-oxidant and anti-cancer properties (8-12). It has been reported that natural compounds have a unique advantage for the treatment of esophageal cancer. They have been shown to enhance immunity, inhibit the growth of cancer cells and reduce cancer relapses and metastases (13,14). Furthermore, natural compounds have been shown to improve the therapeutic efficacy of chemo- and radiotherapy, and reduce their side effects (15). Luteolin, a natural plant flavonoid, obtained from Chrysanthemum morifolium, have been shown to exert an anti-proliferative effect on esophageal cancer cells and induce apoptosis though the mitochondrial pathway (16,17). Berberine has been shown to have potent anti-cancer effects on a variety of cancer cell lines. Berberine has been shown to inhibit the migration and metastasis of esophageal cancer cells (18) and exert anti-proliferative effects by interfering with the mTOR pathway (19). The natural compound, curcumin, has been demonstrated to enhance cancer cell chemosensitivity (20,21), increase cleaved caspase-3 activity, and activate the notch signaling pathway (22).
Magnolol (5,5'-diallyl-2,2'-dihydroxybiphenyl), extracted from a well-known traditional Chinese medicine, Magnolia officinalis, possesses a variety of pharmacological properties, especially anti-cancer properties. Magnolol inhibits the proliferation of human lung squamous carcinoma CH27 cells at low concentrations and induces apoptosis at high concentrations (23). Magnolol inhibits the proliferation, migration, and invasion of hepatocellular carcinoma HepG2 cells in vitro in a dose-dependent manner. In addition, magnolol has been shown to reduce HCC tumor volume and weight in mouse xenograft tumor models (24), and significantly inhibit angiogenesis in vitro and in vivo evidenced by the attenuation of hypoxia and vascular endothelial growth factor (VEGF)-induced tube formation in human bladder cancer cells (25). These findings have led us to investigate the mechanism by which magnolol exerts its anti-cancer activity in esophageal cancer cells.
Methods
Reagents and cell culture
Magnolol (>98% of purity) was purchased from Sigma-Aldrich Co., Ltd. Stock solutions of magnolol were prepared at 100 mM in dimethyl sulphoxide (DMSO) and stored at -80°C. Antibodies for cleaved caspase-3, cleaved -caspase-9, Bcl-2, Bax, JNK, and p-JNK were purchased from Abcam Technology, Inc. Antibodies for GAPDH, cleaved caspase-8, mmp-2, p38, and p-p38 were purchased from Cell Signaling Technology. Anti-ERK and P-ERK were purchased from Santa Cruz Biotechnology. FITC-Annexin V/propidium iodide (PI) apoptosis detection kits and Matrigel were purchased from BD Biosciences. The Caspase-Glo® 3/7 and Caspase-Glo® 9 Assay System were purchased from Promega Corporation. Human esophagus cancer cell lines (TE-1, Eca-109 and KYSE-150) were purchased from the Institute of Biochemistry and Cell Biology (Shanghai Institutes for Biological Sciences, CAS). Cells were maintained in RPMI-1640 supplemented with 10% FBS in a humidified incubator with 5% CO2 at 37 °C.
Cell viability assays
Cell viability treated with different Magnolol concentrations were measured using the CCK-8 kit. Cells were cultured in 96-well plates (1×104/well), and then treated with different concentrations (0, 20, 50, 100 and 150 µM) of magnolol when the cells reached 70–80% confluence. After 24 h or 48 h of incubation, the media was removed and 100 µL CCK-8 buffer was added per well and incubated for an additional 4 h. Absorbance of each well was measured at 450 nm.
Apoptosis analysis
Apoptosis was measured using flow cytometry. FITC-Annexin V/PI detection kit was used to quantify the percentage of cells in different stages of apoptosis. KYSE-150 cells were seeded into 6-well plates, and then treated with PBS (control) or magnolol (20 and 100 µM) for 48 h. Then, 1×105 cells were re-suspended in 100 µL 1× binding buffer. After addition of FITC-Annexin V and PI, the cell suspension was incubated for 15 min in the dark. Subsequently, 400 µL 1×binding buffer was added to the cells for flow cytometry analysis.
Caspase-3 and caspase-9 activity assay
KYSE-150 cells were seeded into 96-well plates at 1×104 cells per well and cultured in complete medium overnight. Cells were then treated with magnolol (0, 20, 50, 100 and 150 µM). Caspase-3 and caspase-9 activity was then measured by adding 50 µL Caspase-Glo® 3/7 or Caspase-Glo® 9 to each well. After a 2 h incubation at 37 °C, luminescence was measured.
Transwell migration assay
Cells were treated with DMSO or 20 µM magnolol for 24 h, and then 1×105 cells were loaded onto a migration chamber. Media containing 10% FBS was placed in the lower chamber. After 12 hours, the cells that had migrated through the membrane were stained using crystal violet. The number of cells that migrated were quantitated using a fluorescence microscope.
Western blotting
Cells were treated with magnolol for 48 h and lysed in RIPA buffer as previously described (26). Lysates were mixed with sample loading buffer and heated to 100 °C for 10 min. After proteins were separated by SDS-PAGE, the samples were transferred to PVDF membranes. Membranes were then blocked with 5% skim milk in TBST and then incubated with the specified primary antibodies overnight at 4 °C. Membranes were then incubated with secondary antibodies for 2 h at room temperature. Protein bands were visualized using enhanced chemiluminescent detection reagent.
In vivo antitumor activity
KYSE-150 cells (1×106) were subcutaneously injected on the right side of the dorsal area of 4-week old female nude mice. Tumor growth was observed daily. Mice bearing tumors were selected and divided into two groups (n=5 each): control group and magnolol (30 mg/kg) group. Magnolol was injected intraperitoneally every other day. Tumor size was measured using calipers, and the tumor volume was calculated using the following formulae; V = 1/2 × (length × width2). All animal procedures were approved by the Institutional Animal Care Committee of Shanghai Changzheng Hospital.
Statistical analysis
Data was expressed as mean ± SEM. Statistical significance was determined using the Student’s t-test or ANOVA analysis. P value of less than 0.05 was considered statistically significant. For all graphs: *P<0.05, **P<0.01, ***P<0.001. All calculations were performed using the SPSS 22.0 software.
Results
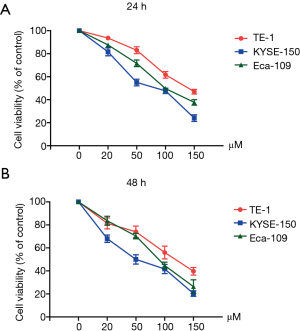
Magnolol inhibits the proliferation of TE-1, Eca-109 and KYSE-150 cells
CCK-8 cell proliferation assay was used to determine the effect of magnolol on esophagus cancer cell proliferation. TE-1, Eca-109 and KYSE-150 cells were treated with different concentrations of magnolol (0, 20, 50, 100 and 150 µM) for 24 h. Magnolol inhibited the proliferation of all three esophagus cancer cell lines in a dose-dependent manner, and had the greatest toxicity on KYSE-150 cells (Figure 1A). To determine whether the inhibitory effect of magnolol on proliferation was time-dependent, we tested the effect using similar concentrations of magnolol for 48 h. The inhibition of proliferation was similar with the 24 h treatment. We observed that the KYSE-150 cell line was the most sensitive to magnolol (Figure 1B), and was selected for future studies.
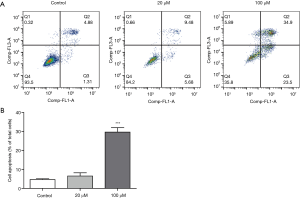
Magnolol induces apoptosis in KYSE-150 cell lines
We further investigated the effect of magnolol on esophagus cancer cell apoptosis using Annexin V-FITC/PI staining. As shown in Figure 2A, a dose-dependent increase in apoptotic cells were observed in magnolol-treated KYSE-150 cells. 20 µM magnolol showed no obvious effect on apoptosis, while 100 µM magnolol treatment significantly increased apoptosis of KYSE-150 cells, P<0.001 (Figure 2B).
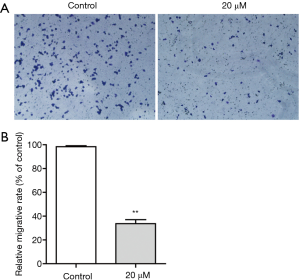
Effect of magnolol on KYSE-150 cell migration
Transwell migration assays were used to measure tumor cell migration. Cells were treated with low dose (20 µM) of magnolol for 24 h, and then transferred (1×105 cells) onto migration chambers. Magnolol treatment significantly reduced cell migration. As shown in Figure 3, 20 µM magnolol markedly inhibited the migration of KYSE-150 cells. Western blot analysis demonstrated that magnolol treatment reduced MMP-2 expression in KYSE-150 cells in a dose-dependent manner. The expression of matrix metalloproteinases (MMPs) family of proteins was closely associated with the migration ability of tumor cells. Magnolol treatment reduced the expression of MMP-2 to affect the migration ability of KYSE-150 cells.
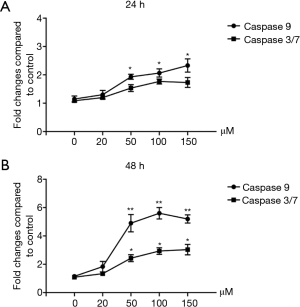
Magnolol activates caspase-3 and caspase-9 in KYSE-150 cells
Caspase-3 and caspase-9 activity was measured using the Caspase-Glo® 3/7 and Caspase-Glo® 9 assay systems. As shown in Figure 4, after 24 h treatment, low-dose magnolol had no obvious effect on the activation of both caspase-3 and caspase-9. However, after a high-dose of magnolol (150 µM) treatment, caspase-9 activity was increased by 2.2-fold and caspase-3 activity was increased by 1.6-fold. After 48 hours, low-dose magnolol (50 µM) could significantly activate both caspase-3 and caspase-9. These results indicate that high dose (150 µM) or 48 hours of low-dose (50 µM) magnolol treatment could induce the activation of the caspase pathway. Activation of the caspase pathway is the major mechanism of cell apoptosis induced by magnolol on KYSE-150 cells.
Magnolol regulates the MAP Kinase pathway
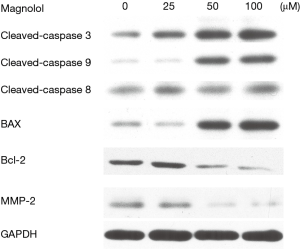
We evaluated the expression of apoptosis-related proteins (cleaved caspase-3, cleaved caspase-8, cleaved caspase-9, Bcl-2, Bax). The results of Western blot analysis showed that magnolol treatment reduced Bcl-2 expression and increased the expression of Bax in KYSE-150 cells in a dose-dependent manner. Furthermore, 50 µM magnolol treatment induced the activation of cleaved caspase-3 and cleaved caspase-9 in KYSE-150 cells while cleaved caspase-8 showed no significant change (Figure 5).
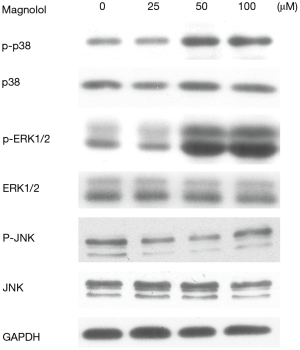
MAPK signaling pathway plays an important role in the regulation of cell apoptosis. To determine whether MAPKs are involved in magnolol-induced apoptosis, we measured the phosphorylation of p38, ERK and JNK by western blot analysis. 50 and 100 µM magnolol treatment increased the phosphorylated form of ERK and p38 as shown in Figure 6. However, phosphorylated JNK was decreased after 25 and 50 µM magnolol treatment. These results indicated that ERK and p38 may play an important role in magnolol induced apoptosis.
Magnolol inhibits the growth of KYSE-150 tumor xenografts
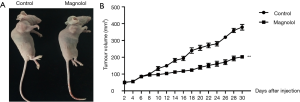
To evaluate the tumor-suppressing effect of magnolol in vivo, a xenograft tumor model using KYSE-150 cells in nude mice was established. KYSE-150 cells (1×106) were subcutaneously injected into 4-week old female nude mice. The effect of magnolol (30 mg/kg) on tumor xenografts was then examined (Figure 7). Magnolol treatment significantly reduced tumor size compared to the controls. Intraperitoneal administration of magnolol (30 mg/kg) reduced tumor volumes by over 50%. These results demonstrated that magnolol treatment effectively reduced growth of KYSE-150 xenografts in vivo.
Discussion
Esophagus cancer is categorized into esophagus squamous cell carcinoma (ESCC) and esophagus adenocarcinoma (EADC). Both subtypes have a high incidence, especially in Eastern Asia, Western America and Oceania (27). Smoking, alcohol and nutrient deficiency are the main risk factors for ESCC, and are commonly observed in developing countries. However, the incidence of EADC are generally observed in developed countries. The risk factors for EADC are age, smoking, obesity and a diet deficient in vegetables (28,29). Overall, the number of ESCC patients are much higher compared to the number of EADC patients worldwide (30). Regardless of the type of esophagus cancer, patients have a poor prognosis and a low quality of life. With the advances in medicine, there are several treatment strategies for esophagus cancer such as surgical resection, chemotherapy, and radiation therapy. However, these treatment methods are inadequate due to their serious side effects, lack of efficacy and tumor recurrence. The development of alternative and safer therapeutic options is vital. Natural compounds are safe, inexpensive, have good efficacy and fewer side effects and are gradually gaining interest and acceptance in the medical community.
Magnolol, a natural compound isolated from the root of magnolia officinalis, has been reported to have anti-oxidative, anti-cancer, anti-inflammatory, and anti-bacterial effects (31-34). However, the effect and mechanism underlying the anti-cancer activity of magnolol for esophageal cancer remains to be deciphered. In this study, we investigated the efficacy of magnolol treatment in human esophagus cancer KYSE-150 cells both in vitro and in vivo. We showed that cellular proliferation decreased significantly after magnolol treatment in a dose- and time-dependent manner.
Caspases are a family of cysteine proteases that play a critical role in apoptosis. When a cell receives an apoptotic signal, cytochrome c interacts with Apaf-1 and procaspase-9 to activate caspase-9. This in turn cleaves and activates caspase-3 and other caspases (35). The cleaved caspase-3 serves as a convergence signal for cell apoptosis. In this study, we found that Magnolol activated caspase-9 and capase-3, and significantly increased their protein expression. However, the levels of cleaved caspase-8 were unchanged.
The Bcl-2 family is composed of multiple members that play an important role in the regulation of apoptosis. We found that magnolol induced the up-regulation of Bax and down-regulated Bcl-2. This is consistent with several previous studies demonstrating the regulation of Bcl-2 family proteins are involved in the apoptosis process (36-38).
The MAPK pathway consists of the p38, ERK and JNK pathway. Our data demonstrated that magnolol induced apoptosis in parallel with the activation of p-ERK and p-38, and the inactivation of p-JNK in esophagus cancer KYSE-150 cells. In xenograft tumor nude mouse models, in vivo treatment with magnolol significantly suppressed tumor growth. Treatment with 30mg/kg of Magnolol reduced tumor volume by over 50%.
Although magnolol had the inhibitory effect on KYSE-150 cell lines, we are unclear on the specific molecular mechanisms of this inhibitory effect. How magnolol exerts its apoptosis function through the MAPK pathway is yet to be deciphered. Magnolol activated both ERK and p38, while it decreased the phosphorylation of JNK. A MAPK pathway specific inhibitor should be used to investigate how magnolol exerts its apoptotic effect.
Conclusions
In summary, our data demonstrated that magnolol inhibited the growth of KYSE-150 cells both in vitro and in vivo by increasing apoptosis. These findings suggest that magnolol may have novel therapeutic benefits for the treatment of esophageal cancer.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal procedures were approved by the Institutional Animal Care Committee of Shanghai Changzheng Hospital.
References
- Ueda H, Takeda M, Ueda S, et al. Clinical evaluation of palliative chemoradiotherapy for metastatic esophageal cancer. Oncotarget 2017;8:80286-94. [Crossref] [PubMed]
- Zhu H, Jin H, Pi J, et al. Apigenin induced apoptosis in esophageal carcinoma cells by destruction membrane structures. Scanning 2016;38:322-8. [Crossref] [PubMed]
- Islamian JP, Mohammadi M, Baradaran B. Inhibition of human esophageal squamous cell carcinomas by targeted silencing of tumor enhancer genes: an overview. Cancer Biol Med 2014;11:78-85. [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Yang CS, Chen X, Tu S. Etiology and prevention of esophageal cancer. Gastrointest Tumors 2016;3:3-16. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Mao WM, Zheng WH, Ling ZQ. Epidemiologic risk factors for esophageal cancer development. Asian Pac J Cancer Prev 2011;12:2461-6. [PubMed]
- Hyuga S, Hyuga M, Oshima N, et al. Ephedrine alkaloids-free Ephedra Herb extract: a safer alternative to ephedra with comparable analgesic, anticancer, and anti-influenza activities. J Nat Med 2016;70:571-83. [Crossref] [PubMed]
- Malongane F, McGaw LJ, Mudau FN. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: a review. J Sci Food Agric 2017;97:4679-89. [Crossref] [PubMed]
- Lin SR, Fu YS, Tsai MJ, et al. Natural Compounds from Herbs that can Potentially Execute as Autophagy Inducers for Cancer Therapy. Int J Mol Sci 2017. [Crossref] [PubMed]
- Ling B, Michel D, Sakharkar MK, et al. Evaluating the cytotoxic effects of the water extracts of four anticancer herbs against human malignant melanoma cells. Drug Des Devel Ther 2016;10:3563-72. [Crossref] [PubMed]
- Li S, Shen XY, Ouyang T, et al. Synergistic anticancer effect of combined crocetin and cisplatin on KYSE-150 cells via p53/p21 pathway. Cancer Cell Int 2017;17:98. [Crossref] [PubMed]
- Liu JB, Yue JY. Preliminary study on the mechanism of oridonin-induced apoptosis in human squamous cell oesophageal carcinoma cell line EC9706. J Int Med Res 2014;42:984-92. [Crossref] [PubMed]
- Wu T, Yang X, Zeng X, et al. Traditional Chinese medicinal herbs in the treatment of patients with esophageal cancer: a systematic review. Gastroenterol Clin North Am 2009;38:153-67. x. [Crossref] [PubMed]
- Ying J, Zhang M, Qiu X, et al. The potential of herb medicines in the treatment of esophageal cancer. Biomed Pharmacother 2018;103:381-90. [Crossref] [PubMed]
- Zhang Q, Zhao XH, Wang ZJ, et al. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol In Vitro 2009;23:797-807. [Crossref] [PubMed]
- Xu H, Yang T, Liu X, et al. Luteolin synergizes the antitumor effects of 5-fluorouracil against human hepatocellular carcinoma cells through apoptosis induction and metabolism. Life Sci 2016;144:138-47. [Crossref] [PubMed]
- Mishan MA, Ahmadiankia N, Matin MM, et al. Role of berberine on molecular markers involved in migration of esophageal cancer cells. Cell Mol Biol 2015;61:37-43. [PubMed]
- Jiang SX, Qi B, Yao WJ, et al. Berberine displays antitumor activity in esophageal cancer cells in vitro. World J Gastroenterol 2017;23:2511-8. [Crossref] [PubMed]
- Hartojo W, Silvers A, Thomas DG, et al. Curcumin promotes apoptosis, increases chemosensitivity, and inhibits nuclear factor κB in esophageal adenocarcinoma. Transl Oncol 2010;3:99-108. [Crossref] [PubMed]
- Tian F, Song M, Xu PR, et al. Curcumin promotes apoptosis of esophageal squamous carcinoma cell lines through inhibition of NF-kappaB signaling pathway. Ai Zheng 2008;27:566-70. [PubMed]
- Subramaniam D, Ponnurangam S, Ramamoorthy P, et al. Curcumin induces cell death in esophageal cancer cells through modulating notch signaling. PLoS One 2012;7:e30590. [Crossref] [PubMed]
- Yang SE, Hsieh MT, Tsai TH, et al. Effector mechanism of magnolol-induced apoptosis in human lung squamous carcinoma CH27 cells. Br J Pharmacol 2003;138:193-201. [Crossref] [PubMed]
- Wang YD, Sun XJ, Yang WJ, et al. Magnolol exerts anticancer activity in hepatocellular carcinoma cells through regulating endoplasmic reticulum stress-mediated apoptotic signaling. Onco Targets Ther 2018;11:5219-26. [Crossref] [PubMed]
- Chen MC, Lee CF, Huang WH, et al. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1α/VEGF signaling pathway in human bladder cancer cells. Biochem Pharmacol 2013;85:1278-87. [Crossref] [PubMed]
- Kang YJ, Park HJ, Chung HJ, et al. Wnt/beta-catenin signaling mediates the antitumor activity of magnolol in colorectal cancer cells. Mol Pharmacol 2012;82:168-77. [Crossref] [PubMed]
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of esophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- Zhang Y. Epidemiology of esophageal cancer. World J gastroenterol 2013;19:5598-606. [Crossref] [PubMed]
- Eslick GD. Epidemiology of esophageal cancer. Gastroenterol Clin North Am 2009;38:17-25. [Crossref] [PubMed]
- Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014;6:112-20. [Crossref] [PubMed]
- Rasul A, Yu B, Khan M, et al. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int J Oncol 2012;40:1153-61. [Crossref] [PubMed]
- Chen JH, Kuo HC, Lee KF, et al. Magnolol protects neurons against ischemia injury via the downregulation of p38/MAPK, CHOP and nitrotyrosine. Toxicol Appl Pharmacol 2014;279:294-302. [Crossref] [PubMed]
- Yang B, Xu Y, Yu S, et al. Anti-angiogenic and anti-inflammatory effect of Magnolol in the oxygen-induced retinopathy model. Inflamm Res 2016;65:81-93. [Crossref] [PubMed]
- Xu HL, Tang W, Du GH, et al. Targeting apoptosis pathways in cancer with magnolol and honokiol, bioactive constituents of the bark of Magnolia officinalis. Drug Discov Ther 2011;5:202-10. [Crossref] [PubMed]
- Shalini S, Dorstyn L, Dawar S, et al. Old, new and emerging functions of caspases. Cell Death Differ 2015;22:526-39. [Crossref] [PubMed]
- Wu H, Medeiros LJ, Young KH. Apoptosis signaling and BCL-2 pathways provide opportunities for novel targeted therapeutic strategies in hematologic malignances. Blood Rev 2018;32:8-28. [Crossref] [PubMed]
- Levy MA, Claxton DF. Therapeutic inhibition of BCL-2 and related family members. Expert Opin Investig Drugs 2017;26:293-301. [Crossref] [PubMed]
- Andreu-Fernández V, Sancho M, Genovés A., et al. Bax transmembrane domain interacts with prosurvival Bcl-2 proteins in biological membranes. Proc Natl Acad Sci USA 2017;114:310-5. [Crossref] [PubMed]


