
Intermediate-term outcomes after aortic valve replacement with a novel RESILIATM tissue bioprosthesis
Introduction
Aortic valve replacement (AVR) in patients with severe aortic stenosis relieves symptoms and increases short term and long survival (1,2). Although bioprosthetic valves are recommended for surgical AVR in patients >60–65 years old, the optimal type of prosthesis in younger patients is less clear (3,4). The excellent durability of mechanical valves may be offset by the need for life-time anticoagulation. However, the use of bioprosthetic valves, particularly in younger patients, is associated with an increased risk of structural valve deterioration (SVD) (5-7).
In an attempt to reduce SVD and improve bioprosthetic durability, a new bioprosthesis tissue platform has been developed (RESILIATM). RESILIATM tissue is made of bovine pericardium that undergoes integrity preservation technology (8). This technology consists of stable capping that permanently blocks calcium (Ca2+) binding sites, and glycerolization that allows dry storage of the bioprosthesis prior to implant (8). The RESILIATM tissue was incorporated within a standard bioprosthesis design and implanted in a cohort of 133 patients who underwent surgical AVR at two centers in Poland. An earlier report of this study through 1 year of follow-up found this bioprosthesis to be safe, and associated with improved hemodynamic performance compared with baseline (9). The current study reports upon the outcomes through an extended follow-up period of 4 years.
Methods
Study design and population
This was a prospective, multicenter, single-arm, observational study (Clinical Trial number: 2010-03, NCT01651052, Clinical Trial of Edwards Aortic Bioprosthesis Model 11000) that was designed to assess the safety and hemodynamic performance of a bioprosthesis developed using a novel tissue platform (RESILIATM). The study protocol was reviewed and approved by the local Ethics Committee (Jagiellonian University Bio-Ethics Committee no. KBET/163/L/2010 of 7 October 2010) and Polish Ministry of Health (CEBK). The study was registered: ClinicalTrials.gov: NCT01651052. All study participants provided written informed consent prior to enrollment. Patients who were >18 years of age and required AVR with or without concomitant procedures such as coronary artery bypass grafting (CABG) were included in the study. The specific inclusion and exclusion criteria have been previously reported (9).
Study device and surgical procedures
Surgical AVR was performed using the Edwards Aortic Bioprosthesis (Model 11000). This tri-leaflet bioprosthesis is the same as the Carpentier-Edwards PERIMOUNTTM Magna Ease aortic valve (Model 3300TFX, Edwards Lifesciences), except for the RESILIATM tissue leaflets. The surgical approach and implantation technique were at the discretion of the investigator and have been reported previously (9). All surgical procedures and implants were performed at the 2 largest cardiac surgery centers in Poland. Patients were offered enrolment into the study by surgeons at individual investigational sites. The decision was based upon an indication for surgical AVR, an appropriate risk profile, and surgical preference for a bioprosthesis. Consenting patients were considered enrolled in the study after the surgeon visually inspected the aortic root, measured the aortic valve annulus, and determined that the study valve could be implanted. In each centre RESILIATM bioprostheses were implanted by two trained surgeons. It was recommended that all patients implanted with the study bioprosthesis be maintained on anticoagulant therapy (except if contraindicated) for approximately 2–3 months based on the American College of Cardiology/American Heart Association (ACC/AHA) 2008 guidelines (4).
Safety and hemodynamic endpoints
The following safety endpoints were evaluated during both the early (≤30 days) and late (>30 days) postoperative periods: all-cause mortality, valve-related mortality, thromboembolism, all bleeding, major bleeding that required transfusion, all paravalvular leak, major paravalvular leak, hemolysis, valve thrombosis, endocarditis, valve explant, non-structural valve dysfunction, and SVD. SVD included dysfunction or deterioration of the implanted valve (exclusive of infection or thrombosis). These safety endpoints were based on objective performance criteria (10), and all events were reviewed and adjudicated by an independent Clinical Events Committee.
Hemodynamic endpoints included the mean and peak systolic transvalvular pressure gradients, the effective orifice area (EOA), and the EOA indexed to body surface area (EOAi). These endpoints were assessed by echocardiography, and all echocardiography data were analyzed by a core laboratory (BioTelemetry Research, Rockville, MD, USA). Patients were assessed preoperatively, at discharge, at 3–6 months, and at 1, 2, 3, and 4 years of follow-up. The preoperative assessments included valve hemodynamic performance, New York Heart Association (NYHA) functional class. These same parameters as well as the safety endpoints were assessed postoperatively, except that hemodynamic measures were not required at the 2- or 4-year follow-up unless murmur was heard on auscultation, and the quality of life measure was collected only at 1 year.
Data management and statistical analysis
As study sponsor, Edwards Lifesciences managed the collection and external monitoring of all data. Additionally, the study was audited and inspected by the Polish Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, with no major findings. Summary statistics for continuous variables are presented as the mean ± SD, unless otherwise noted. Summary statistics for categorical variables include the number and percentage of subjects with a recorded value for the variable of interest. Early safety events were defined as those occurring ≤30 days of the index procedure, and were reported as the number of events divided by the number of enrolled subjects. Linearized rates were used to summarize safety events for the late (>30 day) postoperative period. These rates were calculated as the number of late events divided by the total number of late patient-years. All data are based on an extraction date of April 7, 2017. SAS version 9.3 was used for all statistical analyses.
Results
Baseline characteristics
Between July 2011 and February 2013, a total of 133 patients requiring surgical AVR were implanted with the study valve. The average age of the patients at implant was 65.3±13.5 years, and 26% were ≤60 years old. The proportion of patients with NYHA Class I, II, III, and IV symptoms at baseline was 21.1%, 45.9%, 32.3%, and 0.8%, respectively. Patients underwent AVR for one or more of the following reasons: degenerative valve disease in 93 (69.9%), dystrophic calcification in 24 (18.0%), rheumatic heart disease in 9 (6.8%), endocarditis in 2 (1.5%), and other etiologies in 18 (13.5%). The baseline characteristics of the patients implanted with the study valve are shown in Table 1.
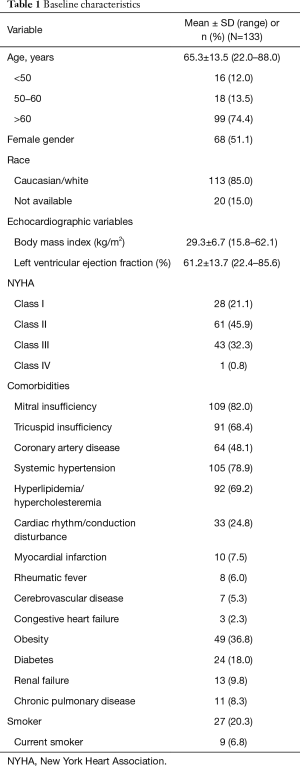
Full table
Procedural outcomes
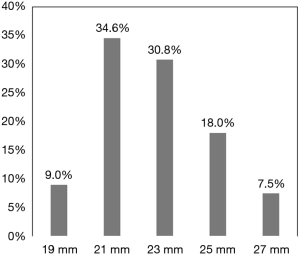
The size of the valves implanted ranged from 19 to 27 mm and are shown in Figure 1. A 19- or 21-mm valve was implanted in 43.6% of the patients. There were 114 patients (85.7%) who underwent isolated AVR, 16 (12.0%) who underwent AVR with concomitant CABG, and 3 (2.3%) who underwent AVR with other procedure(s). The surgical approaches used included a full sternotomy in 117 patients (88.0%) and an upper ministernotomy in 16 (12.0%). Technical success in implanting the study valve was achieved in 100% of patients on the first attempt. The mean aortic cross-clamp and cardiopulmonary bypass times in all 133 patients were 61.7±14.4 and 96.2±25.6 minutes, respectively. In the 114 patients who underwent isolated AVR, the mean aortic cross-clamp and cardiopulmonary bypass times were 59.6±13.1 and 94.5±25.2 minutes, respectively. In the 16 patients who underwent AVR along with CABG, the mean aortic cross-clamp and cardiopulmonary bypass times were 76.9±15.4 and 110.4±25.7 minutes, respectively. The length of stay in the hospital in all 133 patients was 9.7±5.0 days, with 2.2±2.4 days in the intensive care unit and 7.6±5.4 days in the general ward.
Safety outcomes
The average follow-up was 3.8±1.1 (median: 4.1; IQR, 4.0–4.3) years. Early (≤30 days) and late (>30 day) safety outcomes are shown in Table 2. There were 3 (2.3%) cases of all-cause death during the early period and 16 (3.2% late patient-years) during the late period. Valve-related deaths included 1 (0.8%) in the early period and 4 (0.8% late patient-years) in the late period. There was 1 case (0.8%) of major paravalvular leak that required intervention in the early period and none in the late period. The incidence of major bleeding was 6.8% (9 patients) in the early period and 0.4% late patient-years (2 patients) in the late period. One valve was explanted late due to endocarditis. There was also one case of late valve thrombosis that was discovered post-mortem. There were no cases of SVD in the early or late period.

Full table
Hemodynamic outcomes
The echocardiographic data for all subjects stratified by valve size are shown in Table 3. The average mean and peak transvalvular gradients in all patients at 4 years of follow-up were 14.5±7.4 and 26.0±12.9 mmHg, respectively. These gradients were similar to those observed at 3–6 months and represented a marked improvement from baseline (49.4±21.7 and 78.5±32.9 mmHg, respectively). The average EOA improved from baseline (1.0±0.8 cm2) to 3–6 months (1.8±0.5 cm2), and this improvement was also observed at 4 years (1.6±0.4 cm2). The EOAi at 4 years was 0.8±0.2 cm2/m2, and this was improved compared with baseline (0.6±0.4 cm2/m2).
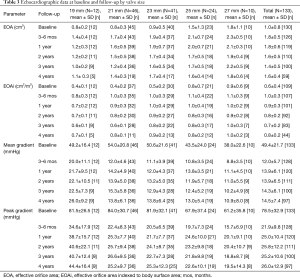
Full table
Functional status and quality of life
The numbers of patients who had NYHA functional assessment at baseline, 1, 2, 3, and 4 years of follow-up were 133, 122, 111, 105, and 101 patients, respectively. Figure 2 shows the changes in NYHA functional class from baseline over the 4-year follow-up period. There was an improvement in NYHA functional class in 55.7% patients at 1 year, 61.3% at 2 years, 56.2% at 3 years and 54.5% at 4 years of follow-up.

Discussion
This trial evaluated the clinical outcomes and hemodynamic performance of an aortic bioprosthesis with the novel RESILIATM tissue over 4 years of follow-up. These results follow-up on the earlier-term safety, durability and hemodynamic performance that have been reported recently (9,11). In another larger recent multicenter observational study, a bioprosthesis with the RESILIA tissue was implanted in 687 patients who needed surgical AVR (12). These patients were followed for approximately 2 years, and the clinical outcomes and hemodynamic performance of the valve were excellent and similar to those observed in the present study.
Although mechanical valves are often recommended for younger patients because they provide superior long-term durability compared to traditional bioprostheses, patients with mechanical valves require life-time anticoagulation, which increases the risk of major bleeding (3,4). Bioprosthetic valves are a reasonable option for patients who wish to avoid long-term anticoagulation; however, these valves are susceptible to SVD, especially in younger patients (5-7,13). The patient-related risk factors for SVD include younger age, increased body mass index, hypertension, diabetes, smoking, dyslipidemia and chronic renal failure; valve-related risk factors for SVD include glutaraldehyde fixation of the leaflets, persistent left ventricular hypertrophy, smaller prosthesis size and prosthesis-patient mismatch (14-17). SVD is thought to occur because the leaflet tissue calcifies over time, resulting in leaflet stiffening or tearing (18).
Several methods of processing leaflet tissue have been developed to try to reduce the amount of tissue calcification (8,19-21). The RESILIATM pericardial tissue undergoes an aldehyde capping process that permanently reduces Ca2+ binding (7,21). This is followed by glycerolization to replace water, allowing dry storage. In an elegant randomized chronic study in juvenile sheep, the RESILIATM tissue exhibited significantly less Ca2+ content and improved hemodynamics compared with the PERIMOUNTTM tissue (22).
The valve hemodynamics reported here highlight the need for consideration of aortic root enlargement in patients with small annuli with valve sizes 19–21 mm. Overall, the study finds an EOA after 4 years of 0.8 cm2/m2, a level typical following surgical AVR with contemporary tissue valves. However, in small valves, the gradients are rather significant, and should serve as another reminder to all surgeons to implement safe as possible means to provide increased EOAs and reduced gradients.
An important finding in the present study is that the prostheses with RESILIATM tissue showed no evidence of SVD over 4 years of follow-up. Although these early results are promising, it must be recognized that SVD is infrequent in the first few years after AVR with a bioprosthesis. In 12,569 patients who underwent AVR using a Carpentier-Edwards PERIMOUNTTM valve, the actuarial estimates of explant for SVD at 10 and 20 years in patients 60-80 years old were 1.5% and 8.1%, respectively (23). Even in patients <60 years old, the actuarial estimate of explant for SVD at 5 years was only 5.6% (23). Thus, the patients in the present study will require a longer follow-up period to confirm the absence of SVD.
Limitations
This trial was a single-arm study without an active comparator group, and the enrollments were not consecutive, though due only to specific inclusion/exclusion criteria. Thus, there may have been selection bias. In addition, the analysis of late outcomes was limited to 4 years of follow-up. Furthermore, not all implanted patients had all data collected at the scheduled follow-ups. A longer follow-up would be required to confirm the findings of this study.
Conclusions
The RESILIATM tissue demonstrated excellent hemodynamic performance and safety outcomes over 4 years of follow-up in the 133 patients enrolled in this trial. Longer follow-up of this patient cohort will be important to confirm the long-term durability of this novel tissue.
Acknowledgments
We thank Trina Patel, PhD, and Lily Jeng of Edwards Lifesciences for statistical support of these analyses.
Footnote
Conflicts of Interest: This study was sponsored by Edwards Lifesciences, LLC. Dr. Bartus is a consultant for Edwards Lifesciences. The other authors have no disclosures to report.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was reviewed and approved by the local Ethics Committee (Jagiellonian University Bio-Ethics Committee no. KBET/163/L/2010 of 7 October 2010) and Polish Ministry of Health (CEBK). All study participants provided written informed consent prior to enrollment.
References
- Brown JM, O'Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [Crossref] [PubMed]
- Litwinowicz R, Bartus K, Drwila R, et al. In-hospital mortality in cardiac surgery patients after readmission to the intensive care unit: a single-center experience with 10,992 patients. J Cardiothorac Vasc Anesth 2015;29:570-5. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [Crossref] [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118:e523-661. [PubMed]
- Stassano P, Di Tommaso L, Monaco M, et al. Aortic valve replacement: a prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J Am Coll Cardiol 2009;54:1862-8. [Crossref] [PubMed]
- Chiang YP, Chikwe J, Moskowitz AJ, et al. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA 2014;312:1323-9. [Crossref] [PubMed]
- Nishida T, Sonoda H, Oishi Y, et al. Long-term results of aortic valve replacement with mechanical prosthesis or carpentier-edwards perimount bioprosthesis in Japanese patients according to age. Circ J 2014;78:2688-95. [Crossref] [PubMed]
- De La Fuente AB, Wright GA, Olin JM, et al. Advanced Integrity Preservation Technology Reduces Bioprosthesis Calcification While Preserving Performance and Safety. J Heart Valve Dis 2015;24:101-9. [PubMed]
- Bartuś K, Litwinowicz R, Kuśmierczyk M, et al. Primary safety and effectiveness feasibility study after surgical aortic valve replacement with a new generation bioprosthesis: one-year outcomes. Kardiol Pol 2018;76:618-24. [PubMed]
- Wu Y, Butchart EG, Borer JS, et al. Clinical evaluation of new heart valve prostheses: update of objective performance criteria. Ann Thorac Surg 2014;98:1865-74. [Crossref] [PubMed]
- Sadowski J, Bartuś K, Kapelak B, et al. Aortic valve replacement with a novel anti-calcification technology platform. Kardiol Pol 2015;73:317-22. [Crossref] [PubMed]
- Puskas JD, Bavaria JE, Svensson LG, et al. The COMMENCE trial: 2-year outcomes with an aortic bioprosthesis with RESILIA tissue. Eur J Cardiothorac Surg 2017;52:432-9. [Crossref] [PubMed]
- Filip G, Litwinowicz R, Kapelak B, et al. Mid-term follow-up after suture-less aortic heart valve implantation. J Thorac Dis 2018;10:6128-36. [Crossref] [PubMed]
- Rodriguez-Gabella T, Voisine P, Puri R, et al. Aortic Bioprosthetic Valve Durability: Incidence, Mechanisms, Predictors, and Management of Surgical and Transcatheter Valve Degeneration. J Am Coll Cardiol 2017;70:1013-28. [Crossref] [PubMed]
- Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg 2005;79:1072-80. [Crossref] [PubMed]
- Filip G, Litwinowicz R, Kapelak B, et al. Patient-prosthesis mismatch after minimally invasive aortic valve replacement. Kardiol Pol 2018;76:908-10. [Crossref] [PubMed]
- Filip G, Bartuś K, Litwinowicz R, et al. Early clinical outcomes of the surgical treatment of patients with aortic stenosis and small aortic annuli. Kardiochirurgia i Torakochirurgia Polska 2013;10:199-203. [Crossref]
- Grunkemeier GL, Furnary AP, Wu Y, et al. Durability of pericardial versus porcine bioprosthetic heart valves. J Thorac Cardiovasc Surg 2012;144:1381-6. [Crossref] [PubMed]
- Chen W, Schoen FJ, Levy RJ. Mechanism of efficacy of 2-amino oleic acid for inhibition of calcification of glutaraldehyde-pretreated porcine bioprosthetic heart valves. Circulation 1994;90:323-9. [Crossref] [PubMed]
- Vyavahare N, Hirsch D, Lerner E, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms. Circulation 1997;95:479-88. [Crossref] [PubMed]
- Shang H, Claessens SM, Tian B, et al. Aldehyde reduction in a novel pericardial tissue reduces calcification using rabbit intramuscular model. J Mater Sci Mater Med 2017;28:16. [Crossref] [PubMed]
- Flameng W, Hermans H, Verbeken E, et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg 2015;149:340-5. [Crossref] [PubMed]
- Johnston DR, Soltesz EG, Vakil N, et al. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg 2015;99:1239-47. [Crossref] [PubMed]


