Anesthetic considerations for bronchoscopic procedures: a narrative review based on the Cleveland Clinic experience
Introduction
The Cleveland Clinic Foundation (CCF) is a tertiary care academic medical center based in Cleveland, Ohio. Since its’ founding the Cleveland Clinic has operated as a multi-disciplinary physician run group practice.
The practice of Interventional Pulmonology started at the Cleveland Clinic on September 20, 1983. Atul Mehta MD, a student of Jean F. Dumon MD, used a Nd-YAG (neodymium-doped yttrium aluminum garnet; Nd:Y3Al5O12) Laser, placed through a bronchoscope, in an anesthetized patient to relieve an airway obstruction in a patient with squamous cell cancer of the lung. With the initiation of the Lung Transplantation program at the CCF in 1990 the portfolio of work expanded to include the various complications that can occur after lung transplantation. Cleveland Clinic was the first institution to introduce successful management of airway dehiscence with temporary placement of uncovered self-expanding metallic stents (1).
The invention of Radial Probe Endobronchial Ultrasound and later Convex Probe Endobronchial Ultrasound resulted in major growth in the department secondary to the ability to stage lung cancer as well as diagnose various lung diseases (2). Despite this new technology, people still had difficulty sampling peripheral lung lesions. This led to the development of electromagnetic navigation bronchoscopy. The Cleveland Clinic was the first center in North America to perform electromagnetic navigation bronchoscopy. The prospective study that was conducted helped to validate and bring acceptance of the technology (3).
Similarly, the Cleveland Clinic has contributed to the development and clinical acceptance of intra-bronchial valves to assist in the treatment of emphysema patients who need lung reduction surgery as well as thermoplasty for the treatment of severe persistent asthma (4,5).
From its inception the Interventional Pulmonary group has practiced in a multidisciplinary fashion including Anesthesiology, Thoracic surgery, Pathology, Radiology as well as Radiation Oncology. The following are the clinical review of the Interventional Pulmonary Anesthesia Group.
Anesthetic technique for advanced bronchoscopy
Recent years have shown an increased demand for anesthesiologists to administer anesthesia as a result of the advent of advanced diagnostic bronchoscopy. Although many of these procedures can be accomplished under moderate sedation, there has been a shift towards general anesthesia. The administration of general anesthesia provides an immobile patient, which is often needed by the pulmonologist and results in higher satisfaction for both, the patient and the pulmonologist.
At our institution the majority of advanced diagnostic, interventional and therapeutic bronchoscopies generally are done one of three ways:
- General anesthesia with a supraglottic airway (SGA);
- General anesthesia with an endotracheal tube (ETT);
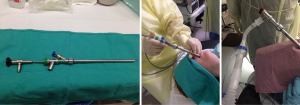
- General anesthesia with rigid bronchoscopy (Figure 1).
Preoperative risk assessment and optimization
Preoperative evaluation as per standard American Society of Anesthesiology guidelines is essential and can help to assess the individual risk and ultimately avoid unexpected intra-procedural complications. Anesthesia providers should focus on procedure related and individualized risk assessment and optimization, instead of wide range testing and assessment. Symptoms and signs related to the primary diagnosis may not be essential for the choice of anesthesia management. For example, obtaining a chest-X-ray in patient with diagnosed chronic obstructive pulmonary disease does not add any specific value to the anesthesia providers. Lung function testing in these patients might be much more appropriate.
Airway assessment is essential, as many pathologies, including tumor diseases might narrow the airway. Presence of stridor prior to procedure may alert to the potential narrowing in the upper airway. Luminal narrowing due to tracheal/bronchial intraluminal or extraluminal compression not only makes airway management difficult or maybe even impossible, but it can also lead to ventilatory difficulties (6). A pre-procedural computed tomography (CT) scan or magnetic resonance imaging (MRI) might help to assess the airway, but should be performed in a timely manner. Upper airway diseases can change quickly and therefore, any scan might not represent the current state of the airway.
For decades, regular and broad laboratory testing was considered the standard of care. However, there is clearly insufficient evidence to identify explicit decision parameters or rules for ordering preoperative tests on the basis of specific clinical characteristics (7,8). Consequently, not least due to financial considerations, laboratory testing should be individualized and focus on relevant testing only. At Cleveland Clinic, we mostly focus on laboratory testing as reported in Table 1.

Full table
Induction of anesthesia and airway management
The routine uses of benzodiazepines and opioids pre procedurally are generally not warranted unless the patient has significant anxiety or pain. One peripheral intravenous line is usually sufficient. Invasive monitoring can be considered if the patient has significant comorbidities. After application of monitors and preoxygenation (target FetO2 ≥0.8), the patient is placed under general anesthesia. Induction agents are at the discretion of the anesthesiologist. Propofol, and lidocaine is commonly used. Bronchoscopy is a stimulating but not a painful procedure thus induction and intraoperative doses of opioids are generally not required.
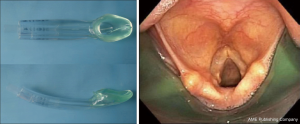
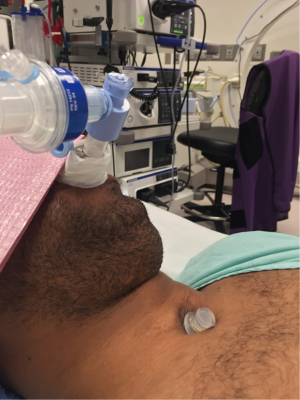
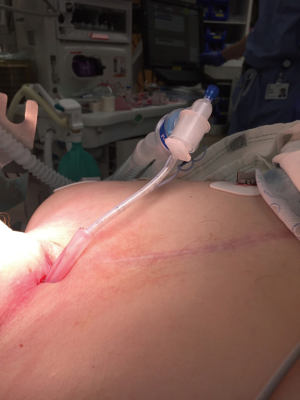
During bronchoscopic procedures, the airway is shared between the anesthesiologist and pulmonologist. The type of airway device used depends on the procedure as well as patient factors. The majority of interventional bronchoscopic procedures performed at our institution are accomplished using a SGA. Our preference is the i-gel (Intersurgical LTD, Berkshire UK) in adequate size (Figure 2). The larger lumen of the i-gel can accommodate the bigger scopes that are used. If an ETT is used, the internal diameter should be above 8.5 or 9 mm to accommodate the larger bronchoscopy scopes and allow simultaneous ventilation. A swivel adaptor is attached to the ETT to enable simultaneous ventilation and bronchoscopy (Figure 3).
Muscle paralysis with a non-depolarizing agent like rocuronium is commonly used in all patients undergoing general anesthesia. Although, SGA placement itself doesn’t need muscle paralysis, paralyzed vocal cords in adduction position facilitates bronchoscopy. Furthermore, muscle paralysis eliminates the risk of coughing and patient’s movements during the procedure.
When rigid bronchoscopy is utilized, the patient is placed under general anesthesia with muscle paralysis. Once muscle relaxation has taken effect, the patient’s airway is handed over to the interventional pulmonologist for insertion of the rigid bronchoscope. Once the scope passes the vocal cords, the anesthesia circuit is connected to the side port of the rigid bronchoscope to initiate ventilation. Significant circuit leak is expected due to the open system nature of rigid bronchoscope. A throat pack made of saline soaked gauze can be inserted around scope. High fresh gas flow up to 15 L/min is routinely utilized to compensate for the circuit leak. Judicious IV fentanyl boluses may be necessary in these cases, not only blunt the sympathetic response from the rigid bronchoscopy stimulation, but also mitigate patient’s cough immediate post procedure.
Maintenance of anesthesia
General anesthesia is maintained with a total intravenous anesthesia (TIVA) regiment using a continuous propofol infusion. This is preferred to volatile anesthetics as the frequent suctioning as well as manipulation of bronchoscopes in and out of patients’ airway inevitably interrupts the delivery of inhalational anesthesia. This results in an inconsistent delivery of the anesthetic to the patient and the potential of intraoperative recall. Secondly, contamination of the operation room from the inhalational anesthesia occurs due to the frequency of leakage. This level of gas leak is worse with rigid bronchoscope than flexible bronchoscopy. When TIVA is used, the integrity of intravenous access should be monitored closely and the Bispectral index monitor is routinely used to monitor the depth of anesthesia (9,10).
Circuit leakage is a common challenge with bronchoscopic procedures. Other than the maneuvers mentioned above, sometime mechanical ventilation needs to be substituted by manual bag ventilation. Alternatively, intermittent apnea can be utilized, however, this technique mandates close monitoring of patient’s vital signs. We maintain our patients on 100% oxygen due to the inconsistent ventilation and frequent leaks.
Other than with rigid bronchoscopy, the use of dexamethasone on most bronchoscopic procedures to decrease airway swelling is controversial and variable in practice.
Emergence from anesthesia
Muscle relaxation is obligatory reversed at the conclusion of the procedure and the patient is emerged from anesthesia. Suctioning is important as there tends to be copious secretions secondary to the irritation of the tracheobronchial tree. This can predispose the patient to laryngospasm, bronchospasm and continued coughing. Indeed, it is common for the patient to cough for some time during their recovery period. The patients generally recover in the bronchoscopy suite recovery room and are discharged home that day.
Specific anesthesia considerations for specific procedures
Endobronchial ultrasound transbronchial needle aspiration (EBUS TBNA)
EBUS TBNA is an advanced diagnostic bronchoscopy technique used for the diagnosis and staging of lung cancer. EBUS TBNA is now used to detect and sample mediastinal and hilar lymphadenopathy as an outpatient procedure, eliminating the need for invasive mediastinoscopy and subsequent hospital admission. EBUS TBNA involves the use of a flexible bronchoscope which also has a linear array ultrasound probe incorporated. This allows the pulmonologist to perform visualization and real time directed needle aspiration of mediastinal lymph nodes and peribronchial lesions. The tissue confirmation obtained by EBUS is critical for the appropriate staging and detection of the histological type of cancer which can then guide treatment.
Initially, pulmonologists used moderate sedation for EBUS exams, but evolving practice now favors general anesthesia (11,12). However the EBUS procedures can be long, requiring a relatively immobile patient and precise positioning, especially for smaller sized nodes or lesions in close proximity to blood vessels. The EBUS bronchoscope itself is of larger diameter than traditional bronchoscopes. For this reason, procedures are generally not well tolerated by patients under moderate sedation (9).
The pulmonologist in our institution prefer an SGA as it allows them access to the entire tracheobronchial tree and mediastinal lymph nodes which may be higher up in the trachea. If an ETT is to be used, it is generally placed shallow in the trachea for the same reason. Muscle relaxation is obligatorily used for these procedures as coughing can result in movement of the mediastinum which may lead to difficulty obtaining an adequate view of the lymph node, inaccurate insertion of the needle and potential injury to mediastinal major vessels (11). Muscle relaxation may make sampling of small paratracheal nodes easier and may decrease the number of sampling attempts and thus shortening the procedure (9).
Initially the airway is examined using the EBUS scope. Then the ultrasound is used to perform ultrasound examination and to guide transbronchial aspiration. The bronchoscope has Doppler capabilities allowing detection and avoidance of mediastina blood vessels. Two screens are available, one to show the fiber optic image of the airway and one to display the corresponding ultrasound image. Generally, lymph nodes greater than 0.5 mm are biopsied. Samples are evaluated there in the bronchoscopy suite by rapid on-site pathology evaluation. This allows verification of adequate samples and preliminary diagnosis (11).
Potential complications of EBUS TBNA are rare but can include pneumothorax, pneumomediastinum, bleeding, laryngospasm, bronchospasm and the other complications of general anesthesia. Key points regarding anesthesia for EBUS TBNA are highlighted in Table 2.

Full table
Electromagnetic navigational bronchoscopy (ENB)
ENB is another technique used to biopsy lesions within the periphery of the lung distal to the bronchoscopic reach. The system comprises of a sensor probe which is able to navigate the bronchial tree and communicate its exact location, an electromagnetic location board, a computer that converts CT scans into multiplanar images with 3-D virtual lung reconstruction and a working channel. It requires a specialized non-magnetic procedure bed. Anesthesia for ENB is similar to anesthesia for EBUS TBNA (9).
Anterior mediastinal mass
Anesthesia for patients with mediastinal masses can be challenging. Anterior mediastinal masses exert a compressive effect on the mediastinal structures, can be extremely hazardous and can lead to complete airway collapse (13-16). Patients generally present with shortness of breath, particularly in the supine position. The major causes of morbidity and mortality are total airway collapse or severe respiratory and cardiovascular complications after induction of anesthesia. All patients with mediastinal masses should be treated with extreme caution, even if asymptomatic.
Patients with mediastinal masses present to the bronchoscopy suite for palliative stenting to relieve compression symptoms or for tumor debulking. The anterior mediastinum is comprised of the sternum anteriorly, the thoracic inlet superiorly, the diaphragm inferiorly and the heart and great vessels posteriorly. Anterior mediastinal masses generally originate from thymomas, lymphomas, teratomas, germ cell tumors, metastatic lesions bronchogenic masses, thyroid tissue or mesenchymal, endocrine and vascular neoplasms (17). These cases are performed under general anesthesia with rigid bronchoscopy or endotracheal intubation.
Pre-operative evaluation
During the pre-operative evaluation of the patient it is essential that the anesthesiologist assess for the severity of symptoms. Symptoms depend on the structures that are being compressed. If the trachea and bronchi are compressed the patient will present with cough and dyspnea. If the superior vena cava (SVC) is compressed, the patient may have SVC syndrome. Compression of the esophagus will present with dysphagia whereas recurrent laryngeal nerve compression will present with hoarseness. If the heart is compressed or if there is effusion or tamponade the patient may complain of syncope or postural symptoms. Identification of the signs and symptoms of the compression, the degree of dyspnea and whether there is a postural component to the dyspnea is important. It is imperative that the position where symptoms are minimized to be established. Positional dyspnea and stridor are ominous signs. Table 3 illustrates the severity of mediastinal masses based on symptoms and imaging.
Other potential hazards caused by mediastinal masses include SVC syndrome, pulmonary artery and cardiac compression. One should evaluate for a decrease in blood pressure from upright to supine position indicative of obstruction to right ventricular filling or ejection. SVC syndrome may present with plethora of head and neck and jugular venous distention (JVD). A pericardial effusion or tamponade may also present with a JVD.
Imaging should include CT scan of the chest, chest X-ray and echocardiogram if heart is involved. Review of the CT scan can give essential information on the position of the mass and compression of surrounding structures.
Anesthetic considerations for mediastinal masses
Patients with mediastinal masses presenting to the bronchoscopy suite are generally symptomatic and need stents placed or tumor debulking to alleviate their symptoms. Patients may present without symptoms of respiratory obstruction; however, induction of general anesthesia can precipitate obstructive symptoms (18). Changing position from sitting to supine has been known to worsen compressive symptoms (19,20). If symptoms are worse in the supine position, all efforts should be made to induce anesthesia in the sitting position.
Beside of airway collapse, other potential acute complications include: SVC obstruction, cardiac tamponade, obstruction to pulmonary artery flow and direct compression of heart (13,21). As a result, strong consideration should be given to placement of a pre-induction arterial line. If SVC syndrome is suspected, placement of a lower extremity intravenous line should be considered.
During spontaneous ventilation, the normal transpulmonary pressure gradient distends the airways and helps to maintain patent airways even in the presence of extrinsic compression. When this distending pressure is lost with the initiation of positive pressure ventilation, the muscles of the chest wall and bronchi relax. Adding to this effect is the bronchial smooth muscle relaxation, decreasing airway size, which occurs with inhalational anesthetic and neuromuscular blockade. There is also a reduction in thoracic volume as a result of cephalad displacement of the paralyzed diaphragm after neuromuscular blockade. The decrease in the size of the airways enhances the effect of extrinsic compression (18,19,21,22). Even without use of neuromuscular blockade, the loss of transpulmonary pressure gradient with positive pressure ventilation can lead to complete obstruction. Thus, difficulty ventilating or major airway obstruction in patients with mediastinal masses can occur during induction, maintenance, positioning or emergence of anesthesia. Due to this potential of complete airway collapse with positive pressure ventilation, maintenance of spontaneous ventilation is of the utmost importance. Awake fiberoptic intubation with the maintenance of spontaneous ventilation should be strongly considered (18,19,21-24). Alternatively, an inhaled induction with volatile anesthesia maintaining spontaneous ventilation with subsequent intubation can be performed. Inhalational anesthesia, ketamine and dexmedetomidine are all good options as they do not ablate respiratory drive. General anesthesia with spontaneous ventilation may be maintained with an inhalational agent or with ketamine or dexmedetomidine infusion (9,25). Ketamine may be safer than inhalational anesthetic with spontaneous respirations as inhalational agents cause a decrease in bronchial wall tone whereas ketamine appears to maintain intercostal and chest wall tone better than inhalational agents (10).
Rigid bronchoscopy may be preferable over endotracheal intubation. The rigid bronchoscope, when placed allows relief of compression and may be preferable for the placement of stents. Placement of a rigid bronchoscope is highly stimulating and may be difficult to place in a spontaneously breathing patient. For rigid bronchoscope placement, sufficient topicalization and deep sedation/general anesthesia with maintenance of spontaneous ventilation is required. If airway collapse occurs, the anesthesiologist should plan for rapid awakening, return of spontaneous ventilation, shifting to rescue position either sitting upright, lateral decubitus or prone. This position needs be determined pre operatively. Also, rapid placement of rigid bronchoscope for ventilation, placed beyond obstruction may be used in the event of complete airway collapse. There may be some consideration for cardiopulmonary bypass if complete airway obstruction is to occur, and should be discussed and arranged pre operatively.
Patients with obstructive airway symptoms, whether extrinsic or intrinsic, have been successfully treated with airway stenting. This allows relief of symptoms for palliative reasons, as a permanent cure or temporary relief of symptoms before radiation treatment is initiated to shrink tumor size (26-29). Key points for the management of mediastinal masses are highlighted in Table 4.

Full table
Tracheal T-tube stent
Patients with T-tubes present for tracheal dilation, removal of granulation tissue and frequent changes of their T-tubes at the bronchoscopy suite. Patients who have persistent tracheal stenosis or malacia have different options for management of their disease, but surgical resection and reconstruction of the diseased area is the best treatment. However patient factors such as medical co-morbidities, or, surgical factors such as the length and areas of stenosis may exclude the patient from having a surgical correction (30). Other options are stenting or permanent tracheostomy. Potential complications of stenting includes stent migration, mucus plugging and granulation tissue formation (31). Tracheostomy is thus often favorable over stenting. The Montgomery T tube was first described over 40 years ago (32). It consists of an uncuffed silicone T-tube that has its long limb in the trachea and its short limb projecting through the tracheostomy stoma. Thus, it has an inferior limb, a superior limb and an anterior limb projecting from the tracheostomy site (33). T-tubes act as stents to maintain airway patency in patients with airway collapse, extrinsic compression, and stenosis or in a post laryngotracheal reconstruction/ healing phase (34). It acts as both a tracheostomy and a stent. It is superior to stenting in that it has decreased rates of migration, allows the ability to suction and the facility to open the cap which allows for an emergency airway should the upper limb become occluded. It is also advantageous over traditional tracheostomy in that it preserves phonation and is cosmetically more appealing. Thus T-tubes are an excellent permanent solution to patients with tracheal stenosis not amenable to surgical reconstruction (35,36).
Airway management for T-tube revision
T-tube change is the most common procedure. Other potential procedures include airway dilation, stoma dilation or stenting of the airway either above or below the stoma. We describe the various management strategies in which the airway is managed for T-tube changes (9,34).
Induction of anesthesia
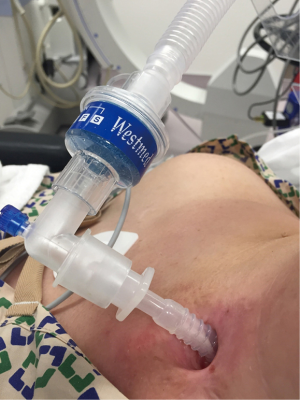
The patient needs to preoxygenated by placing the oxygen mask over the nose and mouth after the T-tube has been capped. Alternatively, the patient can be preoxygenated by attaching the anesthesia circuit to the T-tube. This can be done by attaching an appropriately sized ETT connector to the T-tube and attaching the anesthesia circuit to this connector (Figure 4). Once the patient is asleep and spontaneous breathing stopped, positive pressure ventilation is initiated. If there is excessive leakage from the nose and mouth, this can be alleviated by pinching the nose and lips shut. Although positive pressure ventilation is not 100% effective with this method as the T-tube is uncuffed, it is generally sufficient for a short period of time. Sometimes the placement of a SGA mouth with its connector end capped off, seals the proximal end of the T-tube and decreases leakage through the mouth. At all times anesthesiologists must be vigilant for the occurrence of stomach insufflation as sometimes, especially in situations of non-compliant airways or lungs, there may be excessive leakage of air down into the stomach. Again, anesthesia is maintained with TIVA and the patient is paralyzed with a non-depolarizing neuromuscular blocking agent (37). Choice of airway now depends on the planned procedure.
Placement of a SGA
If the procedure is limited to the anatomic area from the glottis to the tracheostomy site, a SGA is placed (Figure 5). After the T-tube is removed we cover the stoma with petroleum jelly gauze to prevent leakage of gasses through the stoma. A bag of fluid on top of the gauze is used to hold it in place and weigh it down.
Placement of an ETT through the stoma
If the procedure is going to involve more distal intervention, the T-tube is removed and an ETT placed instead and the flexible bronchoscope is introduced through the ETT.
Placement of a rigid bronchoscope
If the procedure is going to involve more distal work on the airways a rigid bronchoscope may be placed through the mouth. Again, the stoma opening is covered with gauze and the mouth packed with saline soaked gauze to minimize leakage. The anesthesia circuit is attached to the side ventilating arm of the rigid bronchoscope and the patient ventilated in this fashion.
Emergence from anesthesia
At the conclusion of the procedure, the patient may be emerged from anesthesia in a number of ways depending on the airway in place.
T-tube
The patient may be emerged from anesthesia by again connecting the newly placed T-tube to the anesthesia circuit via an endotracheal tube connector. Positive pressure ventilation is usually sufficient using this mode for the emergence of anesthesia. If positive pressure ventilation is not sufficient, we place a small cuffed endotracheal tube—size 4, 4.5 or 5, micro laryngeal tube, through the T-tube and inflate the cuff. In this way positive pressure ventilation may be applied (Figure 6). The ETT is then removed from the T-Tube when the patient awakens.
SGA
The patient can be emerged from anesthesia using a SGA. If a rigid bronchoscope was used or if an endotracheal tube was placed in the stoma, and subsequently removed for placement of a new T-tube, the patient may be emerged from anesthesia by placement of an SGA and capping the T-tube.
Bag mask ventilation
Alternatively, the patient may be emerged from anesthesia by assisting ventilation through bag mask ventilation with the anesthesia circuit and mask after capping the T-tube.
Airway hemorrhage/hemoptysis
Airway hemorrhage is a constant challenge faced by the anesthesiologists and the interventional pulmonologists. It may range from clinic non-significant mucosal bleeding to life threatening massive bleeding. The definition of massive hemoptysis varies from 100 to 1,000 mL over a 24-hour period. Keep in mind, that the tracheobronchial tree has a dead space of approximately 150 mL (38-40). Gas exchange impairment from the accumulation of blood in airway is more life threatening than blood loss itself. Patients are more likely to die from asphyxia than from potential hemorrhagic shock.
Airway hemorrhage can be broadly divided into proximal airway bleeding and distal airway bleeding. Proximal airway bleeding is the bleeding from the trachea, main stem bronchi, and proximal lobar bronchi. The distal bleeding is the bleeding from the rest of the bronchial tree, where the bronchoscope may not always be able to reach.
It is common for a small amount of bleeding to occur during regular bronchoscopic procedures. The bronchoscopic interventions to control such bleeding include balloon tamponade, iced saline lavage, topical vasoconstrictor or coagulant application, laser therapy and electrocautery (41-45).
Therapeutic bronchoscopies have significantly higher risk of bleeding than diagnostic bronchoscopies (46). Among the therapeutic bronchoscopic techniques, transbronchial lung biopsy (TBLBs) is an independent risk factor. Clinically moderate to severe bleeding was reported in about 1.1–2.8% of patients undergoing TBLB. The highest risk of bleeding has been reported after cryobiopsies of lung parenchyma, a technique which allows for a higher diagnostic performance in interstitial lung disease (47,48). Compared to TBLB, bronchoalveolar lavage and EBUS-TBNA are considered safe, even in severely thrombocytopenic patients, or patients taking clopidogrel (49).
The vascularization of the tissue sampled is another significant risk factor for bleeding. Biopsies of hypervascularized tumors (especially carcinoids), inflammatory tissue or metastasis (e.g., renal cancers or thyroid cancers) are associated with a higher rate of clinically significant bleeding (50). Accidental biopsy of vascular abnormalities in patients with bronchiectasis or pulmonary artery pseudoaneurysm may cause massive iatrogenic bleeding (51,52). Some hypertrophic dysplastic arteries running in the bronchial submucosa may appear as a non-pulsating endobronchial lesion (53).
Patient-related bleeding risk factors are complicated and should be evaluated. It is not unusual to have patients presenting to bronchoscopic suites already on antithrombotic medication perioperatively. Acetylsalicylic acid has not been shown to increase the risk of bleeding in patients undergoing TBLB, while clopidogrel should be held for 7–10 days before performing invasive bronchoscopic interventions (54). Despite its extreme rarity, massive bleeding does occur and anesthesiologists need to be familiar with the risk factors and the related prophylactic measures (9,55).
When a patient presents with hemoptysis for diagnostic or therapeutic bronchoscopy, it is of paramount importance to protect the nonbleeding lung if the bleeding is still ongoing. If blood spills into the intact lung, the gas exchange will be further compromised. Therefore, a patient should be positioned accordingly to prevent contamination of the non-bleeding lung, i.e., a patient with a right lung bleeding should be placed in the right-side down decubitus position, whereas a patient whose left lung is bleeding should be placed in the left-side down decubitus position (56). Interventional radiology, thoracic surgeon and extra corporeal membrane oxygenation (ECMO) team should be promptly notified.
After positioning, the anesthesiologists need to promptly secure a patent airway. In terms of lung isolation, double lumen tube insertion has some shortcomings. Each of the double lumen tubes’ lumens has a much smaller internal diameter compared to a large bore single lumen endotracheal tube. This limits passage of the therapeutic bronchoscope (57,58). Therefore, a large bore single lumen tube (size 8 or greater, if feasible) combined with a bronchial blocker is a preferred measure (59,60).
If the hemorrhagic source is at the proximal airway, then a rigid bronchoscope is often chosen by interventional pulmonologist (61). It can not only be therapeutic at controlling bleeding by providing a tamponade effect but may also facilitate ventilation by allowing evacuation of large amounts of blood (62). ECMO can be utilized as the last resort when severe refractory hypoxia threatens patient’s life (63). Once patient is stabilized, bronchial artery embolization or thoracic surgery can be considered (64,65). Key points for the management of airway hemorrhage are listed in Table 5.

Full table
Airway stents
Airway stents can effectively restore the central airway patency, especially when no other therapeutic options are available (66,67). The indications for airway stenting are summarized in Table 6. Anatomically, airway stenting is most commonly performed in the trachea, main stem bronchi, or the bronchus intermedius. Airway stenting is often utilized in combination with other common endobronchial interventions, e.g., balloon dilation (Figure 7), electrocautery, laser, argon plasma coagulation etc. (68).

Full table
Silicone and metallic stents are the most common material types. Silicone is a synthetic material created from silicone elastomers or polydimethylsiloxane, with various degrees of firmness and flexibility (69). Silicone stents can be easily modified by cutting a portion of the stent, allowing customization to the airway anatomy prior to deployment. These stents have the advantage of ease of placement, adjustment and removal with low rates of trauma and perforation. The design of the tube also prevents tumor growth into the lumen. Disadvantages of silicone stents include a higher rate of stent migration. Silicone stents also block the normal bronchial wall mucociliary elevator mechanism and can lead to more sputum plugging (70-72). They are comparatively less expensive, easier to remove, and have a long record of safety. The three most common silicone stents are the Montgomery T-tube, the tubular stent and the Y-stent. Most of the time they require a rigid bronchoscope to deploy, which necessitates different anesthesia management compared to metallic stents (73)
Self-expanding metal stents (SEMS) are metal mesh stents which are compacted into an introducer for deployment. Once deployed, the stent opens to expand and tightly fit into the airway. They may be placed under local anesthesia using flexible bronchoscopy or general anesthesia using flexible or rigid bronchoscopy. Because of the snug fit and rapid rate of epithelialization in the airway, migration rates are lower, however further adjustment or stent removal may be more difficult. SEMS have a higher rate of trauma, including pneumothorax, pneumomediastinum, infection, vascular and airway damage as well as bleeding. The SEMS do not block the normal bronchial wall mucociliary elevator mechanism, thus mucus plugging is less of a problem. Tumor and granulation tissue can grow through non covered metal stents and re-occlude the airway (74). This is less of an issue with “covered” SEMS, which are now available.
Three decades have passed since the modern airway stents were developed in 1980s. Interestingly, the practice pattern of metallic stent deployment has had fluctuated more dramatically than that of silicone stents (75). Proceduralists witnessed the initial rapid popularity of metallic stents despite the caution advised by the United States Food and Drug Administration (FDA) in 2005 regarding its usage for benign airway disease (76). However, most recently a resurgence of metallic stents use has surfaced due to both technological advancements and better post stenting care (77).
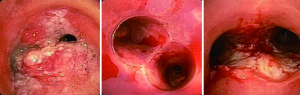
Stents are generally placed for symptomatic central airway obstruction, e.g., airway stenosis or for impairment of the airway integrity, e.g., post lung transplant dehiscence, fistula, etc. (9) (Figure 8). Patients with a stenotic airway should have a detailed history of airway compromise. It is not uncommon that many of the patients need multiple sequential therapies. The documents of previous general anesthesia and intraoperative airway management can be used as important references to formulate anesthetic plan and anticipate any potential intraoperative and immediate postoperative airway complications. Lung transplant patients may often have other significant comorbidities and present more anesthetic challenges.
General anesthesia is often performed as it not only provides a comfortable and amnestic experience for the patient but ensures better operating conditions for the pulmonologist. More importantly, unexpected patient movement during stent deployment can lead to unwanted displacement. If flexible bronchoscopy is planned, either SGA or large-bore ETT can be used with general anesthesia and muscle relaxation (78). When silicone stents are chosen, it is not uncommon that rigid bronchoscope is utilized for deployment (61,79).
In certain situation such as trachea esophageal fistula (TEF) or bronchial wall dehiscence, it may be prudent to avoid positive pressure ventilation. With regards to a TEF, the key anesthetic concerns involve risk of pulmonary aspiration, insufflation of the stomach and hypoxemia and hypercarbia due to loss of tidal volume through the TEF into the stomach. It is therefore important to maintain spontaneous ventilation. Both dexmedetomidine and inhalational anesthetics are good choices as have no significant respiratory depression (80). Meanwhile, in order to minimize patient’s cough, the interventional pulmonologist may judiciously spray lidocaine on the vocal cords and trachea through the bronchoscope.
Vigilance and close communication with interventional pulmonologist are of paramount importance. Compared to the other procedures not involving airway, airway stenting requires anesthesiologist’s constant adjustment to optimize patient’s oxygenation and ventilation. Most importantly, anesthesiologists need to be aware of the rare but potentially lethal complications, specifically massive airway hemorrhage, airway rupture and airway fire when cautery and lasers are used. If the risk of complete airway compromise is suspected, it is prudent to proceed with a multidisciplinary approach with thoracic surgeon and or ECMO stand by or already in place.
Anesthesia for bronchoscopic lung volume reduction valves
Emphysema is characterized by loss of elastic recoil from destruction of alveolar walls and permanent enlargement of airspaces distal to the terminal bronchioles. Air trapping, flattening of the diaphragm and remodeling of the chest to a barrel shaped configuration contribute to increased work of breathing and symptoms of dyspnea.
While medical management with bronchodilators, corticosteroids and oxygen supplementation has been the mainstay of treatment, surgical treatment has emerged as an alternative for patients whose symptoms are refractory to medical therapy. Lung volume reduction surgery typically employs resection of emphysematous portions of the lung to decrease hyperinflation and improve elastic recoil. As morbidity remains high with open or thoracoscopic surgical intervention, interest in viable alternative minimally invasive techniques led to the development of bronchoscopic lung volume reduction techniques.
Currently the United States FDA has approved therapies to include bronchoscopic placement of the duckbill shaped Zephyr Endobronchial Valve and the umbrella shaped Spiration Endobronchial Valve (81,82). The former was approved in July 2018 and the latter was approved in December 2018. Their use is limited by the fact that most insurance companies in the United States still consider their use as “investigational” and do not reimburse their placement without pre-authorization. They have also approved these valves to treat patients with persistent air leaks as studies have demonstrated efficacy in treating both Alveolar-pleural fistulas as well as Bronchopleural fistulas (83).
In general, our pulmonologists prefer that these patients are intubated. Patients can become acutely dyspneic if positioned supine and frequently benefit from preoxygenation and induction of anesthesia while seated in the recumbent position. Post induction patients can be repositioned supine for intubation. With regards to ventilator management, patients often need longer exhalation times to avoid air trapping. Additionally, application of excessive positive end expiratory pressures, light anesthesia and large tidal volumes are avoided as these can increase pulmonary vascular resistance worsening right ventricular function.
Conclusions
We present our anesthetic management for the various advanced diagnostic, interventional and therapeutic bronchoscopic procedures for the patient that we manage in our bronchoscopy suite at the Cleveland Clinic.
Acknowledgments
This narrative review is based on the patient-oriented, effective and collegial collaboration at the bronchoscopy suite at Cleveland Clinic, Main Campus. The authors want to thank all team members from the bronchoscopy suite including physicians, CRNA’s, nurses and technicians for their ongoing support and collegiality.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Mughal MM, Gildea TR, Murthy S, et al. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med 2005;172:768-71. [Crossref] [PubMed]
- Panchabhai TS, Chaddha U, McCurry KR, et al. Historical perspectives of lung transplantation: connecting the dots. J Thorac Dis 2018;10:4516-31. [Crossref] [PubMed]
- Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med 2006;174:982-9. [Crossref] [PubMed]
- Wood DE, McKenna RJ Jr, Yusen RD, et al. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J Thorac Cardiovasc Surg 2007;133:65-73. [Crossref] [PubMed]
- Castro M, Rubin A, Laviolette M, et al. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 2011;107:65-70. [Crossref] [PubMed]
- Goudra BG, Singh PM, Borle A, et al. Anesthesia for Advanced Bronchoscopic Procedures: State-of-the-Art Review. Lung 2015;193:453-65. [Crossref] [PubMed]
- De Hert S, Staender S, Fritsch G, et al. Pre-operative evaluation of adults undergoing elective noncardiac surgery: Updated guideline from the European Society of Anaesthesiology. Eur J Anaesthesiol 2018;35:407-65. [Crossref] [PubMed]
- Ruetzler K, Lin P, You J, et al. The Association Between Timing of Routine Preoperative Blood Testing and a Composite of 30-Day Postoperative Morbidity and Mortality. Anesth Analg 2018;127:897-903. [Crossref] [PubMed]
- Abdelmalak BB, Gildea TR, Doyle DJ. Anesthesia for bronchoscopy. Curr Pharm Des 2012;18:6314-24. [Crossref] [PubMed]
- Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. N Engl J Med 2008;358:1097-108. [Crossref] [PubMed]
- Sarkiss M, Kennedy M, Riedel B, et al. Anesthesia technique for endobronchial ultrasound-guided fine needle aspiration of mediastinal lymph node. J Cardiothorac Vasc Anesth 2007;21:892-6. [Crossref] [PubMed]
- Yarmus LB, Akulian JA, Gilbert C, et al. Comparison of moderate versus deep sedation for endobronchial ultrasound transbronchial needle aspiration. Ann Am Thorac Soc 2013;10:121-6. [Crossref] [PubMed]
- Pullerits J, Holzman R. Anaesthesia for patients with mediastinal masses. Can J Anaesth 1989;36:681-8. [Crossref] [PubMed]
- Frawley G, Low J, Brown TC. Anaesthesia for an anterior mediastinal mass with ketamine and midazolam infusion. Anaesth Intensive Care 1995;23:610-2. [Crossref] [PubMed]
- Dasan J, Littleford J, McRae K, et al. Mediastinal tumour in a pregnant patient presenting as acute cardiorespiratory compromise. Int J Obstet Anesth 2002;11:52-6. [Crossref] [PubMed]
- Slinger P, Karsli C. Management of the patient with a large anterior mediastinal mass: recurring myths. Curr Opin Anaesthesiol 2007;20:1-3. [Crossref] [PubMed]
- Blank RS, de Souza DG. Anesthetic management of patients with an anterior mediastinal mass: continuing professional development. Can J Anaesth 2011;58:853-9,860-7. [Crossref] [PubMed]
- Goh MH, Liu XY, Goh YS. Anterior mediastinal masses: an anaesthetic challenge. Anaesthesia 1999;54:670-4. [Crossref] [PubMed]
- Neuman GG, Weingarten AE, Abramowitz RM, et al. The anesthetic management of the patient with an anterior mediastinal mass. Anesthesiology 1984;60:144-7. [Crossref] [PubMed]
- Prakash UB, Abel MD, Hubmayr RD. Mediastinal mass and tracheal obstruction during general anesthesia. Mayo Clin Proc 1988;63:1004-11. [Crossref] [PubMed]
- Mackie AM, Watson CB. Anaesthesia and mediastinal masses. A case report and review of the literature. Anaesthesia 1984;39:899-903. [Crossref] [PubMed]
- Chen SH, Hsu JC, Lui PW, et al. Airway obstruction by a metastatic mediastinal tumor during anesthesia. Chang Gung Med J 2005;28:258-63. [PubMed]
- Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974;41:242-55. [Crossref] [PubMed]
- Sibert KS, Biondi JW, Hirsch NP. Spontaneous respiration during thoracotomy in a patient with a mediastinal mass. Anesth Analg 1987;66:904-7. [Crossref] [PubMed]
- Galway UA, Doyle DJ, Sable J. Perioperative management of a patient with a massive lipomatous mediastinal mass, severe cardiomyopathy, and tracheal stenosis for urgent laser bronchoscopy and stent placement. J Clin Anesth 2011;23:669-71. [Crossref] [PubMed]
- Miyazawa T, Yamakido M, Ikeda S, et al. Implantation of ultraflex nitinol stents in malignant tracheobronchial stenoses. Chest 2000;118:959-65. [Crossref] [PubMed]
- Shiraishi T, Kawahara K, Shirakusa T, et al. Stenting for airway obstruction in the carinal region. Ann Thorac Surg 1998;66:1925-9. [Crossref] [PubMed]
- Madden BP, Datta S, Charokopos N. Experience with Ultraflex expandable metallic stents in the management of endobronchial pathology. Ann Thorac Surg 2002;73:938-44. [Crossref] [PubMed]
- Rafanan AL, Mehta AC. Stenting of the tracheobronchial tree. Radiol Clin North Am 2000;38:395-408. [Crossref] [PubMed]
- Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-92; discussion 492-3. [Crossref] [PubMed]
- Daumerie G, Su S, Ochroch EA. Anesthesia for the patient with tracheal stenosis. Anesthesiol Clin 2010;28:157-74. [Crossref] [PubMed]
- Montgomery WW. The surgical management of supraglottic and subglottic stenosis. Ann Otol Rhinol Laryngol 1968;77:534-46. [Crossref] [PubMed]
- Ni Chonchubhair A, O'Connor T, O'Hagan C. A novel approach to insertion of the Montgomery T-tube. Anaesthesia 1994;49:605-7. [Crossref] [PubMed]
- Wootten CT, Rutter MJ, Dickson JM, et al. Anesthetic management of patients with tracheal T-tubes. Paediatr Anaesth 2009;19:349-57. [Crossref] [PubMed]
- Saghebi SR, Zangi M, Tajali T, et al. The role of T-tubes in the management of airway stenosis. Eur J Cardiothorac Surg 2013;43:934-9. [Crossref] [PubMed]
- Varvares MA. Tracheal T-tubes for Long-term Management of the Unreconstructable Trachea in Adults. Otolaryngol Head Neck Surg 2017;157:164-6. [Crossref] [PubMed]
- Ramaswamy AH, Kurdi MS. Sindhupriya. TIVA-A Promising Approach to Anaesthetic Management of Montgomery T-tube Insertion. J Clin Diagn Res 2015;9:UD03-4. [PubMed]
- Garzon AA, Cerruti MM, Golding ME. Exsanguinating hemoptysis. J Thorac Cardiovasc Surg 1982;84:829-33. [PubMed]
- Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci 1987;294:301-9. [Crossref] [PubMed]
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008;32:1131-2. [Crossref] [PubMed]
- Valipour A, Kreuzer A, Koller H, et al. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005;127:2113-8. [Crossref] [PubMed]
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980;35:901-4. [Crossref] [PubMed]
- Tsukamoto T, Sasaki H, Nakamura H. Treatment of hemoptysis patients by thrombin and fibrinogen-thrombin infusion therapy using a fiberoptic bronchoscope. Chest 1989;96:473-6. [Crossref] [PubMed]
- Bense L. Intrabronchial selective coagulative treatment of hemoptysis. Report of three cases. Chest 1990;97:990-6. [Crossref] [PubMed]
- Oh S, Peters JI, Folch E. Iatrogenic Endobronchial Bleeding: Speed Things Up or Cool It Down? J Bronchology Interv Pulmonol 2015;22:191-4. [Crossref] [PubMed]
- Zhou GW, Zhang W, Dong YC, et al. Flexible bronchoscopy-induced massive bleeding: A 12-year multicentre retrospective cohort study. Respirology 2016;21:927-31. [Crossref] [PubMed]
- Cooley J, Balestra R, Aragaki-Nakahodo AA, et al. Safety of performing transbronchial lung cryobiopsy on hospitalized patients with interstitial lung disease. Respir Med 2018;140:71-6. [Crossref] [PubMed]
- DiBardino DM, Haas AR, Lanfranco AR, et al. High Complication Rate after Introduction of Transbronchial Cryobiopsy into Clinical Practice at an Academic Medical Center. Ann Am Thorac Soc 2017;14:851-7. [Crossref] [PubMed]
- Stather DR, MacEachern P, Chee A, et al. Safety of endobronchial ultrasound-guided transbronchial needle aspiration for patients taking clopidogrel: a report of 12 consecutive cases. Respiration 2012;83:330-4. [Crossref] [PubMed]
- Cordasco EM Jr, Mehta AC, Ahmad M. Bronchoscopically induced bleeding. A summary of nine years' Cleveland clinic experience and review of the literature. Chest 1991;100:1141-7. [Crossref] [PubMed]
- Pal K, Seeram VK, Cury JD. An unusual case of iatrogenic haemoptysis. BMJ Case Rep 2014;2014. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Patelli M. Life-threatening bleeding after endobronchial biopsy in a patient with bronchiectasis. Am J Respir Crit Care Med 2013;188:e9-10. [Crossref] [PubMed]
- Chawla M, Getzen T, Simoff MJ. Medical pneumonectomy: interventional bronchoscopic and endovascular management of massive hemoptysis due to pulmonary artery pseudoaneurysm, a consequence of endobronchial brachytherapy. Chest 2009;135:1355-8. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68 Suppl 1:i1-44. [Crossref] [PubMed]
- Basem Abdelmalak SS. Thomas Gildea. Anesthesia and Upper and Lower Airway Management for Advanced Diagnostic and Therapeutic Bronchoscopy. Advances in Anesthesia 2014.71-87. [Crossref]
- Yendamuri S. Massive Airway Hemorrhage. Thorac Surg Clin 2015;25:255-60. [Crossref] [PubMed]
- Cohen E. Pro: the new bronchial blockers are preferable to double-lumen tubes for lung isolation. J Cardiothorac Vasc Anesth 2008;22:920-4. [Crossref] [PubMed]
- Klafta JM, Olson JP. Emergent lung separation for management of pulmonary artery rupture. Anesthesiology 1997;87:1248-50. [Crossref] [PubMed]
- Ruetzler K, Grubhofer G, Schmid W, et al. Randomized clinical trial comparing double-lumen tube and EZ-Blocker for single-lung ventilation. Br J Anaesth 2011;106:896-902. [Crossref] [PubMed]
- Schuepbach R, Grande B, Camen G, et al. Intubation with VivaSight or conventional left-sided double-lumen tubes: a randomized trial. Can J Anaesth 2015;62:762-9. [Crossref] [PubMed]
- Flannery A, Daneshvar C, Dutau H, et al. The Art of Rigid Bronchoscopy and Airway Stenting. Clin Chest Med 2018;39:149-67. [Crossref] [PubMed]
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010;80:38-58. [Crossref] [PubMed]
- Lee CF, Huang CT, Ruan SY. Endotracheal tube clamping and extracorporeal membrane oxygenation to resuscitate massive pulmonary haemorrhage. Respirol Case Rep 2018;6:e00321. [Crossref] [PubMed]
- Chen J, Chen LA, Liang ZX, et al. Immediate and long-term results of bronchial artery embolization for hemoptysis due to benign versus malignant pulmonary diseases. Am J Med Sci 2014;348:204-9. [Crossref] [PubMed]
- Alexander GR. A retrospective review comparing the treatment outcomes of emergency lung resection for massive haemoptysis with and without preoperative bronchial artery embolization. Eur J Cardiothorac Surg 2014;45:251-5. [Crossref] [PubMed]
- Ayub A, Al-Ayoubi AM, Bhora FY. Stents for airway strictures: selection and results. J Thorac Dis 2017;9:S116-21. [Crossref] [PubMed]
- Ahuja S, Cohen B, Hinkelbein J, et al. Practical anesthetic considerations in patients undergoing tracheobronchial surgeries: a clinical review of current literature. J Thorac Dis 2016;8:3431-41. [Crossref] [PubMed]
- Gnagi SH, White DR. Beyond dilation: current concepts in endoscopic airway stenting and reconstruction. Curr Opin Otolaryngol Head Neck Surg 2016;24:516-21. [Crossref] [PubMed]
- Karush JM, Seder CW, Raman A, et al. Durability of Silicone Airway Stents in the Management of Benign Central Airway Obstruction. Lung 2017;195:601-6. [Crossref] [PubMed]
- Martinez-Ballarin JI, Diaz-Jimenez JP, Castro MJ, et al. Silicone stents in the management of benign tracheobronchial stenoses. Tolerance and early results in 63 patients. Chest 1996;109:626-9. [Crossref] [PubMed]
- Abdullah V, Yim AP, Wormald PJ, et al. Dumon silicone stents in obstructive tracheobronchial lesions: the Hong Kong experience. Otolaryngol Head Neck Surg 1998;118:256-60. [Crossref] [PubMed]
- Bolliger CT, Probst R, Tschopp K, et al. Silicone stents in the management of inoperable tracheobronchial stenoses. Indications and limitations. Chest 1993;104:1653-9. [Crossref] [PubMed]
- Semaan R, Yarmus L. Rigid bronchoscopy and silicone stents in the management of central airway obstruction. J Thorac Dis 2015;7:S352-62. [PubMed]
- Sihoe AD, Wan IY, Yim AP. Airway stenting for unresectable esophageal cancer. Surg Oncol 2004;13:17-25. [Crossref] [PubMed]
- Dutau H, Dumon JF. Airway Stenting Revisited: 30 Years, the Age of Reason? J Bronchology Interv Pulmonol 2017;24:257-9. [Crossref] [PubMed]
- Lund ME, Force S. Airway stenting for patients with benign airway disease and the Food and Drug Administration advisory: a call for restraint. Chest 2007;132:1107-8. [Crossref] [PubMed]
- Avasarala SK, Freitag L, Mehta AC. Metallic Endobronchial Stents: A Contemporary Resurrection. Chest 2019;155:1246-59. [Crossref] [PubMed]
- Levitan RM, Kinkle WC. Initial anatomic investigations of the I-gel airway: a novel supraglottic airway without inflatable cuff. Anaesthesia 2005;60:1022-6. [Crossref] [PubMed]
- Batra H, Yarmus L. Indications and complications of rigid bronchoscopy. Expert Rev Respir Med 2018;12:509-20. [Crossref] [PubMed]
- Abdelmalak B, Marcanthony N, Abdelmalak J, et al. Dexmedetomidine for anesthetic management of anterior mediastinal mass. J Anesth 2010;24:607-10. [Crossref] [PubMed]
- Criner GJ, Sue R, Wright S, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE). Am J Respir Crit Care Med 2018;198:1151-64. [Crossref] [PubMed]
- Wood DE, Nader DA, Springmeyer SC, et al. The IBV Valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol 2014;21:288-97. [Crossref] [PubMed]
- Venkatappa N, Fadul R, Raymond D, et al. Endobronchial valves for treatment of bronchopleural fistula in granulomatous polyangitis: a longitudinal case report. J Bronchology Interv Pulmonol 2013;20:186-8. [Crossref] [PubMed]