
Off-pump mitral valve repair: primary result of treating moderate ischemic mitral regurgitation during off-pump coronary artery bypass grafting
Introduction
Treating moderate ischemic mitral regurgitation (IMR) in patients with coronary artery disease (CAD) undergoing off-pump coronary artery bypass grafting (OPCAB) is arguable and difficult. Mitral valve repair (MVP) is the first choice in treating moderate-to-severe IMR under cardiopulmonary bypass (CPB). In last decade, percutaneous treatment of mitral regurgitation was used in clinic (1,2). Now we proposed and used off-pump mitral valve repair to treat moderate IMR. The left ventricular surface just below coronary sinus was folded. This would reshape the annulus and the left ventricle below the mitral annulus. Sixteen patients with moderate mitral regurgitation underwent off-pump MVP mentioned previously during OPCAB. The surgical procedure is simple. The early results showed that this way is safe and effective.
Technique
The study was approved by the Ethics Committee of Beijing Anzhen Hospital.
Moderate mitral regurgitation was defined as the mitral regurgitation area in left atrium was 3–6 cm2 by TEE in surgery or 4–8 cm2 by transthoracic echocardiography (TTE). In operation, after finishing OPCAB, the transesophageal echocardiography (TEE) was used to evaluate the severity of IMR and the morphology of mitral valve leaflets and annulus. Patients with triple-branch coronary artery disease (CAD) and with moderate IMR were prepared for off-pump mitral valve repair. Patients with 3-branch CAD and mitral leaflet thickness, leaflet and annular calcification, chordae fracture were excluded from this study.
The surgical procedure is as follows (Figure 1).

Firstly, gauze was used to pad the bottom of the heart, the epicardial fixer was used to totally expose the posterior descending artery (PDA), the coronary sinus (CS) and posterior left ventricular artery (PLA) by pulling the left ventricle cephalad (Figure 2). Secondly, After carefully checking the right coronary artery near to CS by finger touching, the epicardial tissue including fat cushion and part of ventricular muscle from the left side of PDA to the right side of PLA just below the CS was firstly intramurally sutured by using 2/0 polytetrafluoroethylene, with ‘U-shape’ composite pledgets consisting of inner autologous pericardium and outer polyester felt. The length of suture line was about 4–6 centimeters (Figures 2,3). The second stitch was then placed just 3–5 millimeters below the first stitch in the same mode (Figures 2,3). Finally, the suture line was knotted slowly and firmly to fold the posterior wall of left ventricle. The whole process took less than 5 minutes. Then the TEE was used to evaluate the grade of mitral regurgitation.
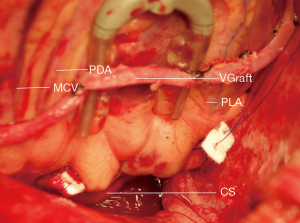
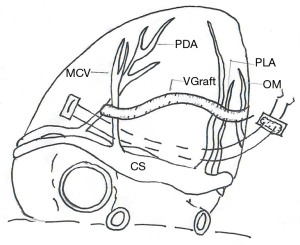
From January 2016 to September 2017, along with OPCAB, off-pump MVP was performed by the same cardiac surgeon in 16 patients whose mean age was 57.4±0.4 years old (11 men, mean age 55 years old, from 38 to 69; 5 women, mean age 66 years old, from 55 to 74). All patients were triple-branch coronary artery disease. When the patients were admitted in hospital, the mitral regurgitation area by TTE was 6.5±1.0 mm2 (4.6–8.0 mm2, all moderate regurgitation). In OPCAB, the number of bypass graft was 3.8±0.4, the pedicled left internal mammary artery—directed to anterior descending artery-was used in 13 patients, great saphenous vein-directed to diagonal branch in 13 patients, directed to obtuse marginal branch and posterior descending artery in 16 patients—was use in all patients. Heparin was used by the dosage of 1 mg/kg. In surgery after OPCAB, the mitral regurgitation area was 3.8±0.9 mm2 (2.7–5.8 mm2, moderate 13, mild 3 cases) before off-pump MVP, and was reduced to 2.3±1.0 mm2 after off-pump MVP (0.7–3.2 mm2, moderate 5, mild 11 cases) (all by TEE). The mitral regurgitation area was reduced to 3.0±1.0 mm2 (1.0–5.0 mm2, moderate 4, mild 12 cases) (by TTE) at discharge and 3.0±0.9 mm2 (1.0–4.2 mm2, moderate 3, mild 13 cases) (by TTE) at the three-month follow-up. There are no in-hospital or out-of-hospital deaths. All patients are in New York Heart Association functional class I in three-month follow-up.
Comment
The pathophysiology of IMR is still incompletely understood. The mechanism of IMR lies in the annular and subvalvular changes rather than leaflet and chordal structure. IMR is more common after inferior rather than that anterior myocardial infarction (4). The three-vessel coronary artery disease can lead to asymmetric ventricular remodeling, most often affect the inferior and lateral left ventricular wall (5). An inferior myocardial infarction is associated with substantial perturbation of the mitral subvalvular apparatus, and an anterior infarction is usually accompanied by mitral annular changes, such as annular dilation (4,6) and more flattening (7). These situation leads to inadequate leaflet coaptation during cardiac systole. Also IMR may be attributable to posterior leaflet interscallop separation which is associated with mitral annular dilation.
IMR is common in CAD patients. Surgical treatment of moderate-to-severe IMR is recommended by the American College of Cardiology/American Heart Association guidelines. The reduction mitral annuloplasty with an artificial ring along with coronary artery bypass grafting (CABG) under cardiopulmonary bypass (CPB) are the clinical standard for treating severe IMR. Whether treating moderate IMR or not in coronary surgery is still arguable (8,9). There is paradoxical evidence that the compound procedure could have a greater improvement in patients’ function or survival benefit at follow-up, but some studies show that treating moderate IMR in CABG leads to improved mitral valve competence, heart function and decreasement in MR grade at short-term follow-up (8,10,11). Recent study shows that CABG plus MVP under CPB might have more complications related to CPB than CABG alone (12). Thus we proposed a new MVP technique without CPB.
The coronary venous structure lies 1 cm above the posterior mitral annulus on the left atrium and goes parallel to the annulus, extending from one mitral commissure to the other commissure. Transcatheter coronary sinus annuloplasty utilizes the proximity of the coronary sinus to the posterior and lateral mitral annulus (1,2). To remodel the mitral annulus, a device is placed in the coronary sinus to make tension that is delivered to the annulus, therefore the mitral annular perimeter is reduced and leaflet coaptation improves. According to the anatomic characteristic and the experience of percutaneous coronary sinus annuloplasty, we shrinked part of left ventricular surface below the coronary venous structure. The surgical location is near mitral posterior annulus. This procedure could make the posterior annulus be concaved to the center of mitral valve. So the flattening annulus partly restores a nonplanar saddle-shaped structure. In the meantime, shrinking the ventricular surface could remodel the subvalvular apparatus and ameliorate leaflet coaptation. In our study, the mitral regurgitation area was reduced from 3.8±0.9 mm2 (moderate 13, mild 3) before off-pump MVP to 2.3±1.0 mm2 after off-pump MVP (moderate 5, mild 11). The mitral regurgitation area was reduced when the patients were at discharge (mitral regurgitation area: 3.0±1.0 mm2, moderate 4, mild 12). The surgical effect of off-pump MVP was still good at 3-month follow-up (mitral regurgitation area: 3.0±0.9 mm2, moderate 3, mild 13). These improvements are attributed to mitral annular and subvalvular remodeling after off-pump MVP, and the blood supplement of the diseased area of left ventricle after OPCAB. The significant reduction in quantitative parameters of mitral regurgitation were seen in patients during follow-up. The improvement of mitral valve function could lead to favorable ventricular remodeling and vice versa. The shrinkage from epicardium in OPMVP is reliable, so we think that the mid-term result of OPMVP will be good.
Thus, the off-pump MVP can be used in patients with moderate or less regurgitation along with OPCAB. The long-term result is at follow-up.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Braun J, van de Veire NR, Klauta RJ, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg 2008;85:430-6. [Crossref] [PubMed]
- Masson JB, Webb JG. Percutaneous treatment of mitral regurgitation. Circ Cardiovasc Interv 2009;2:140-6. [Crossref] [PubMed]
- Li J, Gu C. The PLA and PDA grafts were done off pump. Asvide 2019;6:209. Available online: http://www.asvide.com/watch/32893
- Giga V, Ostojic M, Vujisic-Tesic B, et al. Exercise-induced changes in mitral regurgitation in patients with prior myocardial infarction and left ventricular dysfunction: Relation to mitral deformation and left ventricular function and shape. Eur Heart J 2005;26:1860-5. [Crossref] [PubMed]
- Yu HY, Su MY, Chen YS, et al. Mitral tetrahedron as a geometrical surrogate for chronic ischemic mitral regurgitation. Am J Physiol Heart Circ Physiol 2005;289:H1218-25. [Crossref] [PubMed]
- Watanabe N, Ogasawara Y, Yamaura Y, et al. Geometric differences of the mitral valve tenting between anterior and inferior myocardial infarction with significant ischemic mitral regurgitation: Quantitation by novel software system with transthoracic real-time three-dimensional echocardiography. J Am Soc Echocardiogr 2006;19:71-5. [Crossref] [PubMed]
- Watanabe N, Ogasawara Y, Yamaura Y, et al. Mitral annulus flattens in ischemic mitral regurgitation: Geometric differences between inferior and anterior myocardial infarction: A real-time 3-dimensioal echocardiographic study. Circulation 2005;112:1458-62.
- Yin L, Wang Z, Shen H, et al. Coronary artery bypass grafting versus combined coronary artery bypass grafting and mitral valve repair in treating ischemic mitral regurgitation: A meta-analysis. Heart Lung Circ 2014;23:905-12. [Crossref] [PubMed]
- Mallidi HR, Pelletier MP, Lamb J, et al. Late outcomes in patients with uncorrected mild to moderate mitral regurgitation at time of isolated coronary artery bypass grafting. J Thorac Cardiovasc Surg 2004;127:636-44. [Crossref] [PubMed]
- Fattouch K, Guccione F, Sampognaro R, et al. POINT: Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg 2009;138:278-85. [Crossref] [PubMed]
- Chan KM, Punjabi PP, Flather M, et al. RIME Investigators. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012;126:2502-10. [Crossref] [PubMed]
- Smith PK, Puskas JD, Ascheim DD, et al. Cardiothoracic Surgical Trials Network Investigators. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2014;371:2178-88. [Crossref] [PubMed]


