
Cryobiopsy in the diagnosis of idiopathic pulmonary hemosiderosis: a case report
Introduction
Transbronchial lung cryobiopsy (TLCB) was first described in 2009 (1) and since then it has become a common diagnostic method in interventional pulmonology. Being a safe and cost-effective method, it has been used to diagnose interstitial lung diseases (ILDs) (2,3). It is estimated that its diagnostic yield varies from 75% to 91% (4-6) and it is superior to traditional transbronchial lung biopsy (TBLB) (6,7) in terms of effectiveness. Although not yet as effective as open lung biopsy, its efficacy and safety are unquestionable (8,9). Consequently, it becomes a good alternative to open lung biopsy (10) and is always the first recommended procedure (11). While several interstitial diseases have been diagnosed by histopathological analysis of cryobiopsy samples, there are no studies, which would refer to idiopathic pulmonary hemosiderosis (IPH) confirmed by this diagnostic method. Here, we present what we believe to be the first IPH case to be confirmed by TLCB.
Case presentation
A 54-year-old man was admitted to the pulmonary medicine department for diagnostics of ILD. He reported a reduced ability to exercise for the last 3 years. Since a few months he noted grade 2 dyspnea according to the modified Medical Research Council scale. He additionally complained of coughing up sputum but denied hemoptysis. Five years earlier, the patient had an upper gastrointestinal tract bleeding due to a duodenal ulcer and gastritis resulting in anemia. He was further diagnosed with hiatal hernia with gastroesophageal reflux. He had not been smoking for 12 years but he had a 25 pack-year smoking history.
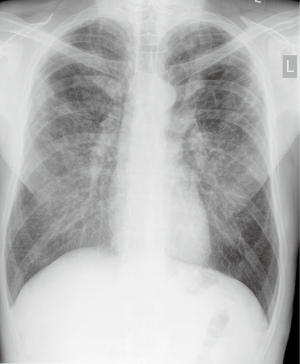
A year earlier, following abnormal chest X-ray findings (Figure 1), the patient had a high-resolution computed tomography (HRCT), which showed bilateral lung emphysema visible especially in upper lobes and coexisting interstitial fibrosis as well as broad interstitial opacities (Figure 2). He was then hospitalized at a regional pulmonology department where some preliminary tests were performed, including bronchoscopy, which did not show any abnormalities. In a 6-minute walk test (6-MWT) the patient walked 530 m with significant desaturation, but spirometry did not confirm any ventilation abnormalities.

On admission to the pulmonary clinic, the patient was in a good condition without significant abnormalities on physical examination. His arterial blood oxygen saturation was 97% at room air. Laboratory tests showed mild leukocytosis with an increased neutrophil level as well as mild normocytic anemia with high reticulocyte count. Following a hematological consultation, the patient was diagnosed with sideropenic anemia and chronic disease anemia due to low blood iron level with concurrent high iron-binding and absorption capability and high ferritin count.
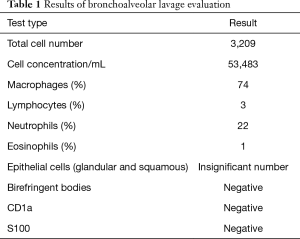
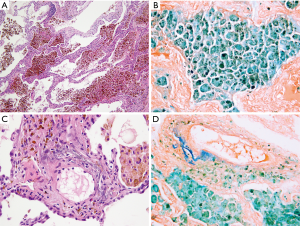
The results of ANA, ANCA, anti-glomerular basement membrane antibodies tests as well as alpha-1 antitrypsin level and test showed no abnormalities. Chest HRCT showed clearly visible interstitial fibrosis. Lung function tests presented lowered CO diffusion capacity single breath transfer factor for carbon monoxide (DLCO SB) was 47.6%) while spirometry and body plethysmography values were normal. In the 6-MWT the patient walked 589 m with significant desaturation. Echocardiography did not show any significant valvular defects and Doppler-measured systolic pulmonary artery pressure was 44 mmHg. Due to the abnormalities in HRCT imaging, the patient was suspected of having pulmonary Langerhans cell histiocytosis but bronchoalveolar lavage (BAL) immunohistochemical analysis of Langerhans cells was negative for S-100 protein and anti-CD1a antibodies. Unexpectedly, BAL showed the presence of hemosiderin-laden macrophages (74%) (Table 1). The patient was admitted for TLCB to an interventional bronchoscopy unit. A radial EBUS mini-probe was used to select the optimal site for biopsy and thus there was no fluoroscopy. Cryobiopsy samples were obtained from two segments of the middle lobe with a 1.9 mm probe (freezing time of 5–8 seconds). In total, there were 5 samples taken. Histopathological examination revealed numerous hemosiderophages (i.e., hemosiderin-laden macrophages) and erythrocytes within alveolar spaces, interalveolar septal thickening and fibrosis as well as alveolar pneumocyte hyperplasia. A delicate incrustation of some vascular walls by iron deposits was noted (Figure 3). There were no features of concurrent vasculitis. Thus an intra-alveolar hemorrhage was diagnosed.
Full table
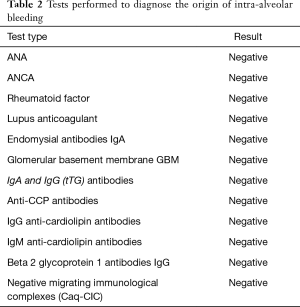
The additional diagnostic tests shown in Table 2 were performed. Considering the result of the microscopic examination of the lung tissue and the broad panel of tests employed which did not show the origin of the intra-alveolar bleeding, the final diagnosis of primary IPH was established.
Full table
Following the diagnosis, the patient was subsequently readmitted to the pulmonary clinic for treatment. On admission he was not in a good condition with no rest dyspnea but peripheral oxygen saturation was 90% at room air. Arterial blood gases showed mild hypoxemic respiratory failure (PaO2 =51 mmHg, PaCO2 =34 mmHg and pH=7.43). Initially, 32 mg/day methylprednisolone per os was introduced. On the third day of treatment, the patient became febrile and had moderate hemoptysis. Chest X-rays showed new lung infiltrates. The C-reactive protein (CRP) level was 210 mg/L. Flexible bronchoscopy revealed fresh bleeding into the bronchial tree. Klebsiella pneumoniae was cultivated at 106 CFU/mL. The patient was diagnosed with hospital acquired pneumonia and diffuse alveolar hemorrhage. Broad-spectrum antibiotic therapy was introduced along with oxygen therapy and 150 mg/day methylprednisolone administered intravenously. As the patient’s condition stabilized after a few days of treatment, azathioprine 150 mg/d was introduced. Methylprednisolone doses were then gradually reduced. After the patient’s condition improved and inflammation regressed, control lung function tests were performed: TLCO SB was at a low but stable level (39%) and in the 6-MWT the patient walked 627 m without significant desaturation.
Azathioprine 150 mg/d was continued and methylprednisolone was gradually reduced (20 mg/day at present). After discharge he was referred for a transplant consultation but was disqualified due to the high risk of relapse observed in IPH patients (12). In the first three months of treatment, the patient reported a significant clinical improvement and he felt even better in the following months. Presently, the patient’s peripheral oxygen saturation at air room is 93% and he does not require long-term oxygen therapy.
In order to broaden the discussion of this case we invited 3 experts from different fields of medicine to participate in the case discussion and answer our questions.
Discussion
Question 1: What is the main advantage of cryobiopsy over the conventional forceps one?
Expert 1 (Antonio Bugalho, expert in interventional pulmonology)
Transbronchial lung cryobiopsy (TBLC) can improve the diagnostic yield in diffuse parenchymal lung diseases especially when clinical and radiological data is insufficient to establish a definitive diagnosis, as in the present case. Current evidence shows that performing two transbronchial lung cryobiopsies from two different segments within the same lobe increases diagnostic yield in diffuse parenchymal lung diseases, nevertheless most centers usually perform 3 to 5 biopsies.
In this case, a 1.9 mm probe was used through the flexible bronchoscope working channel. Commonly, with this cryoprobe, a freezing time of 7 seconds is sufficient to get a good tissue sample but the duration should be adjusted according to the obtained sample size. A radial ultrasound probe was previously used to identify the aimed lung parenchyma and to exclude adjacent blood vessels.
The correct selection of patients is crucial in order to provide an effective diagnosis and diminish complications. This patient had no severe pulmonary hypertension (as defined by a pulmonary arterial pressure of >50 mmHg), forced vital capacity (FVC) <50% or DLCO <35% which are considered relative contraindications for TBLC.
It is a very important point that cryobiopsies have been performed heterogeneously with different strategies regarding the procedure (intubation methods, sedation/anesthesia, probe sizes, freezing time), lung tissue sampling (number of samples, one or multiple lobes, distance to the pleura) and complications (rates, methods to control). Thus, systematization of knowledge in this respect as well as standardization are needed. The TBLB technique is definitely more systematized. However, considering the efficacy of TBLC in the diagnosis of interstitial lung diseases, it is advisable to use this method when TBLB proves insufficient or even as a primary method because it can provide pathologists with considerably better samples for microscope examination.
Expert 2 (Martina Vašáková, clinical expert)
For clinicians, the efficacy and safety of a diagnostic method are of key importance. Sharp’s meta-analysis of 11 studies on TBLB, 11 on TBLC and 24 on surgical lung biopsy (SLB) revealed a diagnostic yield of 64.3%, 84.4% and 91.1% respectively (6). Furthermore, the recent meta-analysis by Iftikhar, comparing TBLC with SLB, showed that diagnostic accuracy of TBLC is comparable with SLB with an acceptable safety profile (9). While we should never forget about individual clinical context, we may agree that TBLC is often the optimal diagnostic method for interstitial lung diseases.
The case describes an ex-smoker with shortness of breath, anemia and dominant emphysema with some interstitial changes at HRCT. Initially, the diagnosis of pulmonary Langerhans cell histiocytosis (PLCH) was suspected, but since the BALF proved inconclusive further diagnostics had to be undertaken. Following the cryobiopsy, the patient was diagnosed with idiopathic pulmonary hemosiderosis (IPH). The CT scan resembles rather that of emphysema or inactive PLCH, since emphysema is not typical for IPH. If it was not for the cryobiopsy performed, the patient would have been misdiagnosed with PLCH.
Expert 3 (Anja C. Roden, specialist in pathomorphology)
The images of this case illustrate and support the advantages of cryobiopsies over conventional forceps biopsies that in general include larger specimen size and well expanded alveoli allowing for easier histologic interpretation.
The cryobiopsy samples show a large piece of lung parenchyma with no crush artifact. I agree that the morphologic findings of large intra-alveolar clusters of hemosiderophages and iron-encrustation of elastic laminas of vessels suggestive of mineralizing pulmonary elastosis are typical for pulmonary hemorrhage. Reportedly, extensive clinical and serologic workup was essentially negative except for a suggestion of pulmonary hypertension given a pulmonary artery pressure of 44 mmHg. There are only a few causes of alveolar hemorrhage that might be recognized morphologically; these include necrotizing vasculitis, pulmonary veno-occlusive disease (PVOD), possibly aspiration, infection, or neoplasms such as angiosarcoma. No morphologic features in this cryobiopsy are suggestive of any of these potential causes. There is some mild interstitial thickening which appears to be due, at least in part, to a mild capillary proliferation, however, the typical morphological features of PVOD such as more pronounced patchy capillary proliferation together with intimal fibrosis of small veins and venules leading at least focally to their occlusion is not appreciated in this specimen. An elastic stain would be helpful to further exclude that possibility. However, many causes of pulmonary hemorrhage have no morphologic correlate and require clinico-radiologic correlation which is necessary before a diagnosis of idiopathic pulmonary hemorrhage can be rendered. It also remains unclear why this patient has pulmonary hypertension.
Question 2: How do you assess the efficacy of cryobiopsy in the diagnostics of IPH on the basis of this case?
Expert 1 (Antonio Bugalho)
I emphasize that TBLC should always be part of a multidisciplinary approach to diffuse lung diseases. When performed by trained professionals in an adequate setting, it may be able to provide good quality histological material that contributes to IPH diagnosis.
Expert 2 (Martina Vašáková)
The case discussed here proves how important cryobiopsy can be for IPH diagnosis. However, cryobiopsy alone should not be considered sufficient because it must be admitted that diagnosis of IPH is per exclusionem and thus it needs to exclude other causes of hemorrhage, mainly vasculitis, antiphospholipid syndrome and anti-basement membrane disease.
Expert 3 (Anja C. Roden)
Histopathological images are not sufficient to diagnose IPH but the information obtained by a pathologist thanks to TBLC can be extremely useful in the differential diagnosis of intra-alveolar hemorrhages and in the final clinical diagnosis.
Conclusions
Idiopathic pulmonary hemosiderosis is a rare disease, which is a result of an alveolar capillary bleeding and it is characterized by a high mortality rate (13,14). The disease is extremely rare in adults (15-18). The clinical course usually suggests intra-alveolar bleeding with characteristic hemoptysis (19) observed in about 80% of patients (15,17,18). In cases similar to the present one, i.e., where there are lung interstitial alterations with a concurrent anemia, IPH should always be considered in the differential diagnosis even though hemoptysis might not be reported by the patient (14,17,19-22). The disease is usually found in children and young adults and thus lung interstitial alterations appear amid a healthy lung tissue. However in adults, comorbidities are more frequently present, also in the case of the respiratory system. Our patient, a former smoker, had pulmonary emphysema further aggravated by concurrent IPH. Consequently, his lung HRCT imaging was equivocal. Flexible bronchoscopy with BAL can also be helpful in diagnostics (15). Apart from newly found bleeding, proof of chronic intra-alveolar bleeding in the form of hemosiderin-laden macrophages is necessary for establishing the diagnosis and this feature has also been found in our patient.
It is known that the progression of idiopathic pulmonary hemosiderosis can be gradual but in some cases the disease can relapse resulting in sudden intra-alveolar bleeding. The risk factors for relapses are not known as such relapses are often spontaneous. However, it seems that in the case of our patient respiratory tract infection could be an important risk factor (16). This is a life-threatening condition and should be treated immediately. Although the use of steroids in such a situation has been questioned (23), the benefits of this therapy cannot be ignored (24). Steroids were applied in the present case and led to clinical improvement of the patient’s condition. Since general practice in severe exacerbations of ILDs is to use steroids with other immunosuppressants, the same has been applied in the present case. Azathioprine allowed to reduce gradually the doses of methylprednisolone. Mesenchymal stem cells (25) can also be used as an additional therapy option. As we had no experience in applying this method, we did not offer it to the patient.
To our best knowledge, there are no case studies on primary IPH diagnosed with TLCB but we observe a growing number of publications describing the usefulness of TLCB based on samples obtained in cryobiopsies (26). This method can be used in cases of interstitial lung diseases, e.g., idiopathic pulmonary fibrosis, to secure diagnostic material for microscopic examination of ILDs when traditional TBLB is usually inadequate, like in IPF (27). The clinical usefulness of TLCB in other ILDs which have been diagnosed previously with open lung biopsy has also already been described in other studies (5). We may thus further confirm the hypothesis that TLCB can be considered an effective method for obtaining a sample suitable for histopathological examination of other rare diseases such as IPH.
In the present case TLCB was uncomplicated and hence, when IPH is suspected, it could be considered as the method of choice before an open lung biopsy. When determining whether to perform TLCB from one or two lung segments, it should be remembered that the latter is more effective (28). Bearing that in mind, we also decided for cryobiopsy from two segments of the middle lobe.
TBLC is a very valuable method for the diagnosis of IPH and, according to the experts bronchoscopy specialist, its use should be recommended because it is safe and effective. It also provides sufficient samples for the pathologist and finally allows the clinical specialist to make a correct diagnosis. Although prognoses are uncertain for IPH, immunosuppressive treatment is a good chance to achieve a stable patient’s condition.
Acknowledgments
None.
Footnote
Conflicts of Interest: M. Szołkowska declares paid lectures for Roche Polska. The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. The signed form is available from the corresponding author upon reasonable request.
References
- Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration 2009;78:203-8. [Crossref] [PubMed]
- Cascante JA, Cebollero P, Herrero S, et al. Transbronchial Cryobiopsy in Interstitial Lung Disease: Are We on the Right Path? J Bronchology Interv Pulmonol 2016;23:204-9. [Crossref] [PubMed]
- Gnass M, Filarecka A, Pankowski J, et al. Transbronchial lung cryobiopsy guided by endobronchial ultrasound radial miniprobe in interstitial lung diseases: preliminary results of a prospective study. Pol Arch Intern Med 2018;128:259-62. [PubMed]
- Bango-Alvarez A, Ariza-Prota M, Torres-Rivas H, et al. Transbronchial cryobiopsy in interstitial lung disease: experience in 106 cases - how to do it. ERJ Open Res 2017;3. [Crossref] [PubMed]
- Kronborg-White S, Folkersen B, Rasmussen TR, et al. Introduction of cryobiopsies in the diagnostics of interstitial lung diseases - experiences in a referral center. Eur Clin Respir J 2017;4:1274099. [Crossref] [PubMed]
- Sharp C, McCabe M, Adamali H, et al. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease-a systematic review and cost analysis. QJM 2017;110:207-14. [PubMed]
- Ganganah O, Guo SL, Chiniah M, et al. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: A systematic review and meta-analysis. Respirology 2016;21:834-41. [Crossref] [PubMed]
- Colby TV, Tomassetti S, Cavazza A, et al. Transbronchial Cryobiopsy in Diffuse Lung Disease: Update for the Pathologist. Arch Pathol Lab Med 2017;141:891-900. [Crossref] [PubMed]
- Iftikhar IH, Alghothani L, Sardi A, et al. Transbronchial Lung Cryobiopsy and Video-assisted Thoracoscopic Lung Biopsy in the Diagnosis of Diffuse Parenchymal Lung Disease. A Meta-analysis of Diagnostic Test Accuracy. Ann Am Thorac Soc 2017;14:1197-211. [PubMed]
- Poletti V, Ravaglia C, Tomassetti S. Transbronchial cryobiopsy in diffuse parenchymal lung diseases. Curr Opin Pulm Med 2016;22:289-96. [Crossref] [PubMed]
- Ravaglia C, Bonifazi M, Wells AU, et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration 2016;91:215-27. [Crossref] [PubMed]
- Calabrese F, Giacometti C, Rea F, et al. Recurrence of idiopathic pulmonary hemosiderosis in a young adult patient after bilateral single-lung transplantation. Transplantation 2002;74:1643-5. [Crossref] [PubMed]
- Ioachimescu OC, Sieber S, Kotch A. Idiopathic pulmonary haemosiderosis revisited. Eur Respir J 2004;24:162-70. [Crossref] [PubMed]
- Bakalli I, Kota L, Sala D, et al. Idiopathic pulmonary hemosiderosis - a diagnostic challenge. Ital J Pediatr 2014;40:35. [Crossref] [PubMed]
- Chen XY, Sun JM, Huang XJ. Idiopathic pulmonary hemosiderosis in adults: review of cases reported in the latest 15 years. Clin Respir J 2017;11:677-81. [Crossref] [PubMed]
- Sherani KM, Upadhyay HN, Sherani FK, et al. Idiopathic pulmonary hemosiderosis presenting in an adult: A case report and review of the literature. Lung India 2015;32:395-7. [Crossref] [PubMed]
- Jillian Cepeda NR. A Rare Case of Idiopathic Pulmonary Hemosiderosis in an Adult. J Pulm Respir Med 2014;4:193. [Crossref]
- Tzouvelekis A, Ntolios P, Oikonomou A, et al. Idiopathic pulmonary hemosiderosis in adults: a case report and review of the literature. Case Rep Med 2012;2012:267857. [Crossref] [PubMed]
- Silva P, Ferreira PG. Idiopathic pulmonary hemosiderosis: Hemorrhagic flare after 6 years of remission. Rev Port Pneumol (2006) 2017;23:368-9. [PubMed]
- Koker SA, Gozmen S, Oymak Y, et al. Idiopathic Pulmonary Hemosiderosis Mimicking Iron Deficiency Anemia: A Delayed Diagnosis? Hematol Rep 2017;9:7048. [PubMed]
- Dogruel D, Erbay A, Yazici N, et al. A Case of Idiopathic Pulmonary Hemosiderosis Presenting With Signs and Symptoms Mimicking Hemolytic Anemia. J Pediatr Hematol Oncol 2017;39:e15-7. [Crossref] [PubMed]
- Chen CC, McManemy JK, Vece TJ, et al. Idiopathic Pulmonary Hemosiderosis Presenting as Anemia, Failure to Thrive, and Jaundice in a Toddler. Pediatr Emerg Care 2016;32:237-9. [Crossref] [PubMed]
- Mushtaq A, Khatoon S, Qureshi MA. Use of Corticosteroids in the management of Idiopathic Pulmonary Haemosiderosis: Do we have enough evidence. Pak J Med Sci 2015;31:487-9. [Crossref] [PubMed]
- Li YT, Guo YX, Cai LM, et al. Methylprednisolone pulse therapy rescued life-threatening pulmonary hemorrhage due to idiopathic pulmonary hemosiderosis. Am J Emerg Med 2017;35:1786.e3-7. [Crossref] [PubMed]
- Xu LH, Ou RQ, Wu BJ, et al. Corticosteroid in Combination with Leflunomide and Mesenchymal Stem Cells for Treatment of Pediatric Idiopathic Pulmonary Hemosiderosis. J Trop Pediatr 2017;63:389-94. [Crossref] [PubMed]
- Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respiration 2018;95:188-200. [Crossref] [PubMed]
- Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic Lung Cryobiopsy Increases Diagnostic Confidence in the Multidisciplinary Diagnosis of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2016;193:745-52. [Crossref] [PubMed]
- Ravaglia C, Wells AU, Tomassetti S, et al. Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Disease: Comparison between Biopsy from 1 Segment and Biopsy from 2 Segments - Diagnostic Yield and Complications. Respiration 2017;93:285-92. [Crossref] [PubMed]


