The prognostic role of angiolymphatic invasion in N0 esophageal carcinoma: a meta-analysis and systematic review
Introduction
Esophageal carcinoma is the 9th most common cancer worldwide with an obviously increasing incidence over the past few decades. The incidence and mortality rate of esophageal carcinoma continues to rise (1). The overall 5-year survival rate of these patients ranges from 15% to 34% because most are only diagnosed in an advanced stage, although multimodality therapy such as surgical resection, endoscopic resection, and preoperative and/or postoperative adjuvant therapy are in practice (2,3). Most patients who undergo curative surgery will relapse and die due to this cancer. The prognostic factors of esophageal carcinoma include histology type, grade category, lymphovascular invasion, differentiation, tumour size, depth of invasion, lymph node metastasis and distant metastasis (4).
Angiolymphatic invasion (ALI) is usually defined as the presence of tumour cells within lymph-vessels and/or blood-vessels. ALI plays a key role in lymph node metastasis and cancer cell spread and it is thought to increase the possibility of micro-metastatic risks in local-regional cancer (5). Our previous meta-analysis proved that ALI was an indicator of a poor prognosis in esophageal carcinoma. This conclusion was more convincing when more studies with a high proportion of early-stage patients were included (6). The possibility of lymph node metastasis is relatively low in early-stage esophageal carcinoma. The independent prognostic effect of ALI in node-negative esophageal carcinoma (N0-EC) could only be demonstrated based on the surgical pathological results of esophagectomy plus adequate lymph node dissection. Herein, we conducted a meta-analysis to add to the evidence on the prognostic significance of ALI in N0-EC.
Methods
Search strategy
We searched the PubMed, EMBASE, Web of Science and Cochrane Library databases for relevant articles. The terms we used are: “lymphovascular invasion”, “lymph vessel invasion”, “angiolymphatic invasion”, “lymphatic invasion”, “vascular invasion”, “blood vessel invasion”, “esophageal cancer”, “esophageal carcinoma”, “node negative”, “N0”, “survival”, “prognosis”. A manual search was used for potentially eligible studies if necessary. Initial screening was implemented by browsing titles and abstracts. Full-text review was an essential step in identifying eligible studies. In our study, ALI included lymphatic invasion (LI), vascular invasion (VI) and angiolymphatic invasion/lymphovascular invasion.
Exclusion and inclusion criteria
Eligible studies included in this review are based on the following inclusion criteria: (I) a study of patients diagnosed with esophageal cancer; (II) reported sufficient survival data such as hazard ratio (HR) with 95% confidence intervals (CI) or the ability to calculate these measures from the presented data; (III) papers published in English; (IV) reported prognostic information about ALI; (V) patients were negative for lymph node metastasis; (VI) the survival data was from multivariable analysis. If multiple studies were carried out by the same authors or group, the newest or the most informative study was selected.
Exclusion criteria included: (I) duplicate report, letter, abstract, conference paper, molecular biology research or review; (II) studies concerning animal models, treatment methods and other types of cancer; (III) survival data were not available; (IV) studies not published in English; (V) included patients with gastric or esophagogastric junction cancer (EJC); (VI) the survival data reported were from univariate analysis.
Initial review of studies and quality assessment
All articles were independently reviewed by two authors (A.W. and YL. T.) to determine the exclusion and inclusion criteria. A third author (SH. W.) was introduced to resolve any discrepancies between the initial review authors. The Newcastle-Ottawa scale (NOS) was used to evaluate the quality of the included articles (7). All articles included scored at least 5 points on the NOS.
Data extraction
Data from the included studies were collected independently by 2 authors (D.X. and YC. F.). The following information was extracted: first author surname, patient follow-up time, study region, study sample size, patient characteristics, histology type, staining methods adopted for detecting ALI, number of patients with ALI, and survival information. All collected information is shown in Table 1. Disagreements were discussed and resolved by a third author (SH. W).

Full table
Statistical analysis
We investigated the association between ALI and prognostic outcomes including overall survival (OS), disease-free survival (DFS) and cancer-specific survival (CSS) after surgery. The effect size used in this meta-analysis was the HR with 95% confidence intervals (CI) from multivariate analysis. A HR >1 indicates a poor prognosis. We used the HR with 95% CI if the article provided it. If the value of the HR with 95% CI was not provided, we calculated the HR and 95% CI from the Kaplan-Meier survival curves using the software Engauge Digitizer Version 4.1 (http://markummitchell.github.io/engauge-digitizer/). The HR could be estimated by the method proposed by Tierney et al. (16). If the study provided a HR and p value, we could calculate the 95% CI. The heterogeneity of the pooled HR with 95% CI was tested by Cochrane’s Q test (Chi-squared test; Chi2) and I2 metric (I2 <25% means no heterogeneity; I2 =25–50% indicates moderate heterogeneity; I2 =50–75% indicates medium heterogeneity; I2 >75% indicates extreme heterogeneity). If I2 <50% with P>0.05, a fixed-effect model (Mantel Haenszel method) was appropriate. Otherwise, a random-effects model was adopted for the analysis. Subgroup analysis was used to explore and explain the heterogeneity when necessary (17). Any publication bias was detected by Begg’s test. All P values were based on two-sided tests, and P≤0.05 was considered statistically significant. Stata/SE 12.0 for Windows (Stata Corporation, College Station, TX, USA) was used to perform the statistical analysis. All data processing was verified by a statistician (YY. Z).
Results
Characteristics of the studies
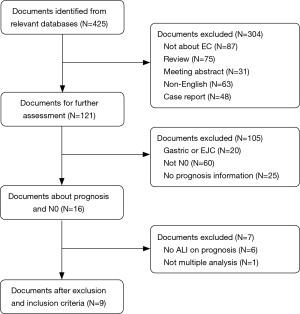
A total of 425 articles were retrieved after the elimination of duplications. After initial review of the title and abstract, articles were excluded for the following reasons: 304 due to their irrelevance for ALI and prognosis: 87 articles due to their irrelevance for EC; 75 articles were in the form of a review; 31 articles were in the form of a meeting abstract; 63 articles were not published in English; and 48 articles were in the form of a case report. Thus, 121 potential articles were retained after the initial review. Then, after further review, 105 articles were excluded for the reasons given below: 20 articles were on esophagogastric junction cancer (EJC); 25 articles did not provide detailed survival information; and 60 articles included patients with lymph node metastasis. Thus, a total of 16 articles were subjected to the full-text review. However, 6 articles failed to provide survival information about ALI, and 1 article was excluded because of the use of univariate analysis. Then, the 9 remaining articles involving 2,154 patients (median: 206, range: 83–627) were selected for our meta-analysis (Figure 1). The detailed information of the selected studies is displayed in Table 1. All studies included in this meta-analysis scored at least five points on the NOS.
The effect of ALI on DFS, OS and CSS
The heterogeneity in the data for both CSS and OS were 0% (CSS: P=0.333; OS: P=0.858). The heterogeneity in the DFS was 53.3% (P=0.073). For CSS, the pooled HR (HR =2.54; 95% CI: 1.84–3.51; P<0.001) showed that patients with ALI have a poor cancer specific survival (Figure 2A). For OS, the pooled HR (HR =2.84; 95% CI: 2.17–3.72; P<0.001) showed that ALI was an indicator of a poor prognosis for OS (Figure 2B). In DFS, the pooled HR was 2.84 (95% CI: 1.85–4.37; P<0.001), which showed that patients with ALI have a shorter disease-free survival (Figure 2C).

Subgroup analysis for DFS
Two subgroup analyses were carried out on DFS according to the study region and median age of the patients in the study. The heterogeneity slightly decreased in subgroup analysis based on the median age <65 (HR =2.35, P=0.024; I2=50.7%, P=0.154, Figure 3A). For the subgroup analysis by study region, with the only non-China study eliminated, the heterogeneity disappeared in the Chinese subgroup (HR =2.51, P<0.001; I2=0.0%, P=0.454, Figure 3B).

Publication bias of the included studies
Begg’s test showed no evidence of publication bias for CSS (P=0.317, Figure 4A), OS (P=0.624, Figure 4B) or DFS (P=0.327, Figure 4C).

Discussion
Our previous meta-analysis proved that ALI was an indicator of a poor prognosis in esophageal carcinoma. This conclusion was more convincing in early-stage EC patients (6). Therefore, we conducted this meta-analysis to evaluate the significance of ALI for predicting the survival of patients with N0 EC. In the current study, a total of 9 studies with 2,154 patients were included. At present, our work is believed to be the first systematic review on the connection between ALI and the prognosis of patients with N0-EC. The pooled HR showed that patients with ALI have a poor CSS (HR =2.54; 95% CI: 1.84–3.51; P<0.001), a poor OS (HR =2.84; 95% CI: 2.17–3.72; P<0.001) and a shorter DFS (HR =2.84; 95% CI: 1.85–4.37; P<0.001). The heterogeneity in the DFS could be explained in the subgroup analysis by the region the study was conducted in.
Some meta-analyses have proven the prognostic role of ALI in prostatic cancer, bladder cancer, and lung cancer (18-20). Our previous meta-analysis has already suggested an association between ALI and prognosis in esophageal carcinoma (6). Lymphatic vessels play an important role in immune modulation, cancer stem cell survival, and the promotion of tumour cell recruitment to lymph nodes (21). Lymphatic vessels promote lymph node metastasis by accumulating tumour cells (22). Lymph node metastasis indicates a significant risk of a poor prognosis in esophageal cancer patients. The role of ALI in lymph node metastasis has been verified in breast cancer by a meta-analysis (23). Some studies have already discussed the relationship between ALI and lymph node metastasis in esophageal carcinoma. There is a high probability of lymph node metastasis in esophageal carcinoma patients with ALI (24-26). Our previous study (8) demonstrated a more significant risk of ALI in early Stage I and II patients. Cen et al. (25) reported that T1bN0M0 esophageal cancer patients with ALI had a similar 5-year OS compared to T1b patients who had lymph node metastasis. Moreover, the 5-year survival rate of T1b esophageal cancer patients without ALI was similar to T1a patients and was longer than the survival rate of T1b cancer patients with ALI. Wang et al. (9) drew a similar conclusion, namely, that patients with ALI had an OS that was similar to N1 patients. Therefore, the prognostic role of ALI independent of lymph node metastasis is worthy of being studied.
ALI is usually defined as the presence of tumour cells in arterial, venous, or lymphatic vessels evident on the pathological evaluation of specimens. The evidence of blood vessels invasion is an erythrocyte in an endothelium line and a thick vessel wall. However, lymphatic invasion is characterized by cancer cells wandering within the endothelium line, which is not present in vascular invasion (10). Many studies have not shown a good distinction of the lymphatic vessels from the blood vessels (5,27-31). Therefore, we attributed LI, VI and ALI/LVI to ALI.
The current meta-analysis justified the significance of ALI in the prognosis of patients with N0-EC. The presence of ALI is an indicator for adjuvant therapy in endometrial cancer (32). ALI has an adverse impact on patients with N0-EC. Could ALI be an indicator for adjuvant therapy in N0-EC patients? The role of postoperative adjuvant chemotherapy in esophageal carcinoma with lymph node metastasis has been established by the clinical trial of the Japanese Clinical Oncological Group (JCOG) 9204 (33). Approximately 30–60% of patients with N0-EC who undergo surgery alone experienced a fatal recurrence within 5 years (34). Patients with esophageal carcinoma and positive LI can also benefit from postoperative adjuvant chemotherapy with low-dose cisplatin (DDP) plus 5-fluorouracil (5-FU) (35). Additionally, ALI is an indicator of an adverse prognosis in patients with N0-EC and has a close connection with lymph node metastasis. Therefore, we could assume that ALI is an indicator for adjuvant therapy after surgery in patients with N0-EC.
Neoadjuvant chemoradiotherapy (nCRT) before oesophagectomy improved survival significantly compared with oesophagectomy alone for locally advanced esophageal cancer patients in the CALGB 9781 trial (36). The benefit of nCRT followed by surgery compared to surgery alone has also been verified by the largest RCT (CROSS) (37). Chen et al. (38) demonstrated that the existence of ALI independently predicted a shorter OS in EC patients receiving nCRT. Their results suggested that patients with ALI receiving nCRT should be taken into consideration regardless of lymph node status. nCRT can downstage tumours, reduce the volume of the mass and decrease the risk of metastasis. ALI is believed to develop prior to LNM. The presence of ALI may predict the necessity of nCRT.
There were also some limitations in this meta-analysis. First, the studies included were restricted to papers published in English. This may lead to some potential bias. Second, tumour stage, study region and staining methods were not completely the same across the studies. In particular, study region was the main cause of heterogeneity. Third, some studies only provided survival curves, so we had to calculate the HR and 95% CI. This could lead to inaccuracy of the HR. Fourth, only one of the studies included patients with adenocarcinoma, so the proportion of patients with adenocarcinoma vs. SCC could not be reported in this paper. Therefore, the conclusion of this paper cannot be applied to adenocarcinoma patients.
In summary, according to the results of our meta-analysis, ALI is an indicator of a poor prognosis in patients with N0-EC. ALI may be useful for identifying high-risk patients that can benefit from multi-modality therapy to further improve the survival of patients with N0-EC.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Shah MA. Future Directions in Improving Outcomes for Patients with Gastric and Esophageal Cancer. Hematol Oncol Clin North Am 2017;31:545-52. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119-30.
- Huang Q, Luo K, Chen C, et al. Identification and Validation of Lymphovascular Invasion as a Prognostic and Staging Factor in Node-Negative Esophageal Squamous Cell Carcinoma. J Thorac Oncol 2016;11:583-92. [Crossref] [PubMed]
- Wang A, Tan Y, Geng X, et al. Lymphovascular invasion as a poor prognostic indicator in thoracic esophageal carcinoma: a systematic review and meta-analysis. Dis Esophagus 2019;32. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Wang S, Chen X, Fan J, et al. Prognostic Significance of Lymphovascular Invasion for Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2016;23:4101-9. [Crossref] [PubMed]
- Wang Z, Chen P, Wang F, et al. Lymphovascular invasion as an independent prognostic indicator in radically resected thoracic esophageal squamous cell carcinoma. Thorac Cancer 2019;10:150-5. [Crossref] [PubMed]
- Imamura Y, Watanabe M, Nagai Y, et al. Lymphatic vessel invasion detected by the D2-40 monoclonal antibody is an independent prognostic factor in node-negative esophageal squamous cell carcinoma. J Surg Oncol 2012;105:277-83. [Crossref] [PubMed]
- Chen GQ, Tian H, Yue WM, et al. SIRT1 expression is associated with lymphangiogenesis, lymphovascular invasion and prognosis in pN0 esophageal squamous cell carcinoma. Cell Biosci 2014;4:48. [Crossref] [PubMed]
- Kunisaki C, Makino H, Oshima T, et al. Clinicopathological features in N0 oesophageal cancer patients. Anticancer Res 2010;30:3063-9. [PubMed]
- Tachezy M, Tiebel AK, Gebauer F, et al. Prognostic impact of perineural, blood and lymph vessel invasion for esophageal cancer. Histol Histopathol 2014;29:1467-75. [PubMed]
- Zhu CM, Ling YH, Xi SY, et al. Prognostic significance of the pN classification supplemented by vascular invasion for esophageal squamous cell carcinoma. PLoS One 2014;9:e96129. [Crossref] [PubMed]
- Jeon JH, Lee JM, Moon DH, et al. Prognostic significance of venous invasion and maximum standardized uptake value of (18)F-FDG PET/CT in surgically resected T1N0 esophageal squamous cell carcinoma. Eur J Surg Oncol 2017;43:471-7. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- Huang Y, Huang H, Pan XW, et al. The prognostic value of lymphovascular invasion in radical prostatectomy: a systematic review and meta-analysis. Asian J Androl 2016;18:780-5. [Crossref] [PubMed]
- Mollberg NM, Bennette C, Howell E, et al. Lymphovascular invasion as a prognostic indicator in stage I non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg 2014;97:965-71. [Crossref] [PubMed]
- Tian YF, Zhou H, Yu G, et al. Prognostic significance of lymphovascular invasion in bladder cancer after surgical resection: A meta-analysis. J Huazhong Univ Sci Technolog Med Sci 2015;35:646-55. [Crossref] [PubMed]
- Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest 2014;124:922-8. [Crossref] [PubMed]
- Das S, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci 2008;1131:235-41. [Crossref] [PubMed]
- Zhang S, Zhang D, Yi S, et al. The relationship of lymphatic vessel density, lymphovascular invasion, and lymph node metastasis in breast cancer: a systematic review and meta-analysis. Oncotarget 2017;8:2863-73. [PubMed]
- Xue L, Ren L, Zou S, et al. Parameters predicting lymph node metastasis in patients with superficial esophageal squamous cell carcinoma. Mod Pathol 2012;25:1364-77. [Crossref] [PubMed]
- Cen P, Hofstetter WL, Correa AM, et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer 2008;112:1020-7. [Crossref] [PubMed]
- Brücher BL, Stein HJ, Werner M, et al. Lymphatic vessel invasion is an independent prognostic factor in patients with a primary resected tumor with esophageal squamous cell carcinoma. Cancer 2001;92:2228-33. [Crossref] [PubMed]
- Jiao XL, Chen D, Wang JG, et al. Clinical significance of serum matrix metalloproteinase-13 levels in patients with esophageal squamous cell carcinoma (ESCC). Eur Rev Med Pharmacol Sci 2014;18:509-15. [PubMed]
- Ren P, Yu ZT, Xiu L, et al. Elevated serum levels of human relaxin-2 in patients with esophageal squamous cell carcinoma. World J Gastroenterol 2013;19:2412-8. [Crossref] [PubMed]
- Raja S, Rice TW, Goldblum JR, et al. Esophageal submucosa: the watershed for esophageal cancer. J Thorac Cardiovasc Surg 2011;142:1403-11.e1. [Crossref] [PubMed]
- Yamashina T, Ishihara R, Nagai K, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol 2013;108:544-51. [Crossref] [PubMed]
- Schoppmann SF, Jesch B, Zacherl J, et al. Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer. Surgery 2013;153:526-34. [Crossref] [PubMed]
- Croog VJ, Abu-Rustum NR, Barakat RR, et al. Adjuvant radiation for early stage endometrial cancer with lymphovascular invasion. Gynecol Oncol 2008;111:49-54. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Shiozaki A, Yamagishi H, Itoi H, et al. Long-term administration of low-dose cisplatin plus 5-fluorouracil prolongs the postoperative survival of patients with esophageal cancer. Oncol Rep 2005;13:667-72. [PubMed]
- Rice TW, Ishwaran H, Hofstetter WL, et al. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus 2016;29:897-905.
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Chen WH, Huang YL, Chao YK, et al. Prognostic significance of lymphovascular invasion in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol 2015;22:338-43. [Crossref] [PubMed]