Robotic-assisted minimally invasive esophagectomy: past, present and future
Introduction
Esophageal cancer presents a significant oncological burden; it is the 7th most common cancer and the 6th most fatal globally (1). Esophageal resection is the mainstay of curative management, achieving a 5-year survival rate that approaches 50% when preceded by neoadjuvant chemo(radio)therapy (2,3).
The surgical approach to the esophagus, especially in the context of esophageal cancer, is challenging due to several factors. Firstly, the esophagus spans both the chest and the abdomen, which typically means that both compartments are accessed to perform an esophagectomy with two-field lymphadenectomy (i.e., dissection of both abdominal and thoracic lymph node stations). This surgical approach is widely preferred worldwide, as it has been shown to increase disease free survival in patients with proven nodal involvement (4,5). Secondly, the esophagus is nestled between vital structures which are not readily resectable; the aorta, atrium, main airways and pulmonary vessels. The need to access the thorax has fuelled a desire to perform esophagectomy by less invasive ways in an attempt to reduce morbidity associated with thoracotomy. The requirement to perform delicate yet thorough dissection around vital thoracic structures without compromising resection margins can be challenging when using standard minimally invasive tools. By offering technical advantages such as enhanced three-dimensional vision and full dexterity, robotic assistance can be useful during complex surgical procedures. This article reviews the origins of minimally invasive esophagectomy (MIE) and robotic-assisted minimally invasive esophagectomy (RAMIE). The available evidence for MIE and RAMIE will be reviewed and future developments will be discussed.
The past
The history and development of MIE are important in order to understand the roots of RAMIE. MIE was first described in 1992 (6) and has seen a steady increase in uptake over the past decades (5,7,8). The first step toward MIE was by means of a laparoscopic transhiatal approach (9). Subsequently true minimally invasive Ivor Lewis or McKeown procedures were developed involving a laparoscopic abdominal phase combined with a thorascopic chest phase. Concerns about the thoracoscopic component with regards to its safety, standardisation and reproducibility across centres led to the development of the “hybrid” esophagectomy consisting of an open chest phase whilst maintaining a laparoscopic abdominal phase.
To date, only one multi-centre randomised trial has compared total MIE to open esophagectomy for distal esophageal tumors; the TIME trial (10). Four nations participated in this seven-centre study randomising a total of 115 patients. MIE consisted of a laparoscopic abdominal phase followed by a right thoracoscopic phase in the prone position. Comparison was made to open esophagectomy, which could involve either a two-stage (i.e., Ivor Lewis) or three-stage (i.e., McKeown) procedure. The primary outcome was pulmonary complications, although definitions, particularly of pneumonia, were not standardised and the relatively high rates of reported pulmonary complication, particularly in the open group (36%) were questioned (11,12). Our group developed and validated the Universal Pneumonia Score in an attempt to standardise post esophagectomy hospital-acquired pneumonia (13). Although controversial with regard to its primary endpoint, the TIME trial showed that MIE was superior to open esophagectomy in terms of postoperative pulmonary infections (relative risk 0.30, 95% CI: 0.12 –0.76; P=0.005). In addition, MIE was associated with less intraoperative blood loss, a better preserved acute immunological response, lower postoperative pain scores, shorter length of hospital stay, and improved quality of life. Long term oncological outcomes have since been reported and show equivalence to open esophagectomy in terms of disease free and overall survival (14).
Multiple systematic reviews comparing MIE to open esophagectomy have been performed over the years, which largely mirror the findings of the TIME trail (15,16). Moreover, several trails are currently recruiting comparing MIE to open esophagectomy to further assess safety, peri-operative morbidity and long-term outcomes. A multicentre, prospective, randomised, open and parallel controlled trial in China aims to compare the effectiveness of MIE to open McKeown esophagectomy for resectable esophageal cancer. It is expecting to recruit 324 patients to each arm over a 3-year period (17).
The MIRO trial was the first to compare open two-stage esophagectomy (laparotomy and right thoracotomy) with hybrid two-stage esophagectomy (laparoscopic abdominal phase and open thoracic phase) (18). The primary end point was 30-day morbidity [grade II–IV on the Clavien-Dindo system (19)]. This trial recruited 207 patients from 12 centres and showed a reduction in major post-operative morbidity (OR 0.31, 95% CI: 0.18–0.55; P<0.001) with equivalent 3-year oncological outcomes (20). In the UK, the ROMIO study is currently recruiting (21). Originally intended to be a three-arm trial comparing open esophagectomy, hybrid esophagectomy (laparoscopic abdomen and open thoracotomy) and total MIE, a lack of standardisation for MIE across centres ultimately resulted in the trial comparing hybrid esophagectomy to open alone.
Whilst the results of MIE are promising, population-based studies in the United Kingdom, Japan, the United States of America, and the Netherlands demonstrated increased re-intervention rates (22-25) after MIE. Some authors postulated that this was possibly an effect of the learning curve that was experienced by surgeons and centres during the early national adaptation phase. This may be a plausible explanation, as the MIE learning curve can be associated with additional morbidity (26) and takes 50–119 cases, depending on the chosen parameters of proficiency. Regardless, these data represent the outcomes in the countries of inclusion and should therefore be carefully considered. As such, follow-up studies are warranted to investigate whether the re-intervention rate has normalized in the more recent years (26-28).
The early evidence that MIE was safe and at least equivalent to open esophagectomy drove the development of RAMIE, aiming to overcome some of the inherent technical difficulties commonly experienced during MIE, particularly the thoracoscopic phase. The relatively rigid chest cavity, with limited access due to the proximity of the ribs, scapula and vertebral column, proved a challenging environment for standard minimally invasive tools. The first RAMIE was performed in 2003 (29) and case series were published in 2006 (30).
RAMIE has become an established technique for performing esophagectomy for resectable esophageal cancer, with groups reporting their experiences all over the world [for example (31-33)]. The term “RAMIE” should be treated with some caution as it is used interchangeably to describe totally robotic esophagectomy (34) or a laparoscopic abdominal phase combined with a robotic thoracic phase (35). Alternative terms such as robotic assisted Ivor Lewis esophagectomy (RAILE), robotic assisted transhiatal esophagectomy (RATE), robotic assisted mini invasive McKeown esophagectomy (RAMIME) and robotic Ivor-Lewis esophagectomy (RILE) are also used in the literature. Equally the location and method of performing the oesophagogastric anastomosis varies between centres which has resulted in heterogeneous groups making interpretation and comparisons of case series difficult. A systematic review of all 16 available case series showed that a majority of patients had a cervical anastomosis, although nearly half of the studies did not specify the type of construction used to form the join (36). At this stage the literature consisted entirely of case series but proved the initial feasibility and safety of RAMIE with good short-term oncologic results compared to both MIE and open esophagectomy.
The present
Current evidence
To date >600 RAMIE procedures have been performed in the University Medical Centre Utrecht for esophageal cancer. We have recently published a single centre, superiority, controlled, parallel-group, randomized controlled trial comparing RAMIE to open McKeown esophagectomy; the ROBOT trail (35). This was the first trial to compare open esophagectomy to RAMIE and showed a lower percentage of overall surgery-related and cardiopulmonary complications in the RAMIE group with lower postoperative pain, better short-term quality of life, and a better short-term postoperative functional recovery without compromising oncological outcomes (37).
Sarkaria et al. recently published the results of a single centre comparative study (34). Here, open esophagectomy (either Ivor Lewis or left thoracoabdominal procedures) was compared to RAMIE (robotic thoracic and abdominal phases, almost exclusively Ivor Lewis) with the primary outcome being Quality of Life (QoL) as assessed by the Functional Assessment of Cancer Therapy-Esophageal (FACT-E) subset and the Brief Pain Inventory (BPI). This was a non-randomized comparative study where allocation to treatment was determined by which surgeon the patient presented to. Although the cohorts were not propensity matched, the baseline characteristics were not statistically different. The results showed that short-term QoL was better following RAMIE. The study also assessed oncological outcomes and peri-operative morbidity as secondary outcomes. As was described in the ROBOT trial, the authors showed that pulmonary complications were lower in the RAMIE group. Beyond this, they reported a reduction in infective complications, a reduction in re-admission to ICU and an increase in lymph node yield in the RAMIE group.
Both van der Sluis et al. (35) and Sarkaria et al. (34) have shown that RAMIE is safe and results in reduced peri-operative morbidity, improved early QoL, with equivalent oncological outcomes compared to open esophagectomy. To date, only one study has compared MIE to RAMIE (robotic abdomen and thorax) (32). In this single surgeon, propensity matched study 66 patients were paired to compare MIE with RAMIE. The authors found that, apart from a longer operative time for RAMIE, the outcomes, both oncological and in terms of complications, were equal. A commentary (38) on the study commended the clinical relevance of this study, but raised the issue that in a cohort of total of 76 patients (from which 66 were propensity matched) the learning-curve effect, which takes 20–70 cases for RAMIE, may have affected this study. The REVATE study will be the first multi-centre, open-label, randomized controlled trial to prospectively compare RAMIE to MIE (39). This trial is based on a single surgeon experience (40), which showed reduced recurrent laryngeal nerve neuropraxia in the RAMIE group with a minimal learning curve effect of 12 procedures for experienced thoracoscopic surgeons. Although the primary outcome will be recurrent laryngeal nerve neuropraxia, secondary outcomes will include complication rates and oncological outcomes.
Precision surgery
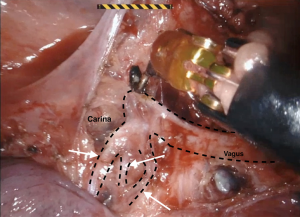
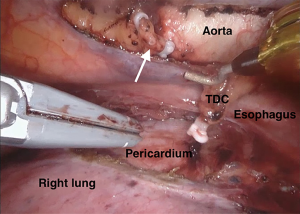
The current robotic platforms allow 10-fold magnification and a stable, three-dimensional endoscopic view. As a result of this, we were able to identify anatomy not previously recognised or documented. For example, our group collaborated to first describe a distinct fascial layer surrounding the esophageal blood supply and lymphatics in essence forming a “meso-esophagus” (41,42). We also identified that the branches contributing to the thoracic duct join approximately 7 cm superior to the esophageal diaphragmatic hiatus (43). The magnified, stable, view during RAMIE routinely allows us to clip the thoracic duct en bloc and visualise a single transected lumen (Figure 1). A further anatomical detail, that is more readily appreciated and preserved during RAMIE, are the vagal branches supplying the right and left main bronchus (44,45) (Figure 2). The fact that several studies have now shown reduced pulmonary complications in RAMIE is likely to be multifactorial. One of these factors may be related to the ability to preserve the parasympathetic innervation of the lungs by sparing vagal branches which has been associated with reduced pulmonary complications (46).
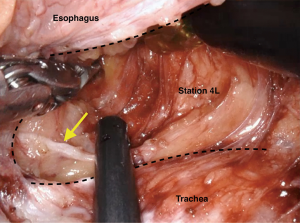
We routinely perform upper mediastinal lymphadenectomy (nodal stations 4 and 2, bilaterally) in the thorax as previously described (47) (Figure 3). Studies have shown that even in the context of distal esophageal cancers (Siewert Type I) these stations contain nodal metastases in 11% of cases (48). Again, the use of the robot has given greater access in terms of operating in both the aperture of the chest whilst maintaining the ability to reach the hiatus (this is a limitation in thoracoscopic surgery), as well as the ability to visual and spare the recurrent laryngeal nerves whilst dissecting paratracheal lymph node stations.
With the developments in robotic technology, multi-quadrant work has become more attainable. This is of particular relevance in the abdomen, where the required dissection spans a relatively large filed from the apex of the greater curve, to the duodenum, to the splenic hilum and the diaphragmatic hiatus. The use of the da Vinci Xi in particular has aided uptake in upper GI robotic surgery, which is reflected by the recent increase of reported total robotic-assisted gastrectomies. Beyond this, advances in energy dissecting tools now make the dissection in the abdomen, particularly along the greater curve and pylorus of the stomach, safer, faster and more haemostatic. As a result, several centres, amongst these our own centre, have started to routinely perform fully robotic esophagectomies (31,34).
The future
The concept of sentinel lymph node identification and sampling is well established in oncological surgery (49-51). In esophageal cancer, however, this has proved challenging with the initial use of blue dyes (52,53). Advances in in radio-guided techniques using peritumoral injection of 99mTc antimony colloid by upper endoscopy prior to the operation has proven to have a high sensitivity and specificity in identifying sentinel nodes in early esophageal cancer (54), although similar techniques have been less useful in advanced cancers (55). In the context of a 23% complete response rate in adenocarcinomas following neo-adjuvant treatment with CROSS (3) or radical endoscopic resections for T1 tumors, this technology could be crucial in avoiding the significant morbidity associated with esophagectomy and 2-field lymphadenectomy; in patients with cT1N0M0 middle or lower esophageal cancer, if sentinel nodes are detected in the mediastinum or abdominal but are all histopathologically negative for cancer metastasis, lymphadenectomy may potentially be omitted. We are currently recruiting to the SNAP study (Sentinel node Navigation surgery in early Adenocarcinoma Patient) (56) which uses a combination of ICG an Tc99 which is injected in the tumor the day prior to surgery in an attempt to identify sentinel nodes in the context of cT1N0M0. The ability for the operating surgeon to readily switch between fluorescence and plain light modes whilst maintaining a stable magnified view and permitting selective nodal dissection is greatly aided by robotic systems.
Oncological treatments are becoming increasingly progressive in treating oligometastatic disease and previously deemed irresectable tumors. There are reports of locally advanced tumors invading the aorta, atrium, pulmonary vessels, or the airways (i.e., T4b disease) being successfully treated by means of down staging through radical chemoradiotherapy followed by RAMIE (57,58).
The future of RAMIE is closely related to the developments in robotic platforms. In terms of the available hardware, multiple new systems are expected over the next couple of years (59,60). Recently, improvements in robotic tri-stapling devices, energy dissection instruments and Firefly integration have streamlined RAMIE. In the near future, the greatest future advances in robotic surgery, however, are likely related to software developments. The use of artificial intelligence, data and imaging integration and connectivity will open up new possibilities in terms precision surgery, but also permit big data collection and machine learning (61).
Training in complex minimally invasive surgical techniques such as 2-stage esophagectomy is a long process with learning curves from 50 to 119 cases described (26,28). A recent meta-analysis suggested as many as 36 anastomotic leaks in a series of 646 should be directly attributed to the learning curve (26). Equally, early series in MIE have reported higher rates of acute gastric conduit necrosis (28), which in some cases were attributed to technique and put down to the learning curve effect. Interestingly, the learning curve of RAMIE is reported to be 20–70 (57). The observation that this may indeed be lower is not simply a reflection of the fact that these surgeons progressed from MIE to RAMIE, since some of the reported series went straight from open esophagectomy to RAMIE. However, it should be noted that the parameters of proficiency and chosen methods to visualize the learning curve varied in previous studies, which makes it impossible to draw any firm conclusions regarding the length of the learning curve for RAMIE in relation to MIE. The key is likely to be the implementation of a structured training programme which has proven benefits in significantly lowering the learning curve of a procedure irrespective of the parameters of proficiency selected. The availability of dual console systems has further enhanced the teaching and training opportunity in the context of RAMIE; in our unit we have divided the thoracic and abdominal phases into their constituent parts and use a global assessment scale ranging from 1–6 (where 1 is “step done by trainer” and 6 is “masterful performance by trainee”) to grade the performance and required proctoring of the trainee surgeon. Advances in digital connectivity, particularly through 5G networks, have allowed the realistic developments in telesurgery—remote operating, where the console surgeon and patient are not within the same vicinity. Although not a new phenomenon, the first tele-surgical cholecystectomy was performed in 2001 (62), the increasingly accepted concept of remote operating will potentially play a significant part in the proctoring process.
The advent of single port robotic systems (e.g., da Vinci SP system, Intuitive Surgical Inc, SPORT surgical system, Titan Medical Inc) will inevitably see developments in single port stages of either the abdominal or thoracic (or both) phase(s) of esophagectomy. The single port robotic systems can be of particular significant in the cervical approach for the upper mediastinum. To date, most surgeons perform the transcervical approach of the transhiatal esophagectomy by blunt dissection. This potentially limits the quality of the lymph node dissection. The single port mediastinoscopic cervical approach has been described in order to improve esophageal dissection and lymph node harvesting of the upper mediastinum in transhiatal esophagectomies (63,64). However, dissecting with straight, conventional, non-articulating instruments within the narrow deep mediastinal working place around the aortic arch and tracheal bifurcation is challenging. The single site robotic platform can overcome these challenges. The wristed 3D camera and fully wristed elbowed instruments allow surgeons to increase surgical quality and thus expand its applications in the mediastinum. A preclinical study has demonstrated the feasibility of a transcervical esophagectomy with the da Vinci SP (65). This study described excellent visibility and handling of the mediastinal organs located along the esophagus. Several groups have already reported on single incision thoracic phases, although these were not performed robotically (66-68). The current single port robotic systems are still limited with the absence of a vessel sealing device or robotic staplers. Until the single port robotic systems are embedded and standardized, it is difficult to consider the additional value in the transthoracic phase compared to the multiport robotic systems.
Concluding remarks
To date, esophagectomy and lymphadenectomy remains a cornerstone in the treatment of esophageal malignancy in combination with chemo(radio)therapy. Minimally invasive methods for the oncological resection of the esophagus have equivalent long-term oncological outcomes, but reduced peri-operative morbidity and improved QoL compared to open surgery. Several studies have now shown that RAMIE is safe, feasible and results in reduced complications compared to open surgery. Evidence from randomized prospective trials comparing MIE and RAMIE is awaited. The expected influx of new robotic platforms including the use of artificial intelligence will impact robotic surgery through even less invasive surgery, big data sharing, augmented reality, and adjunctive technology such as the use of ICG to routinely assess perfusion, localise lymphatics or identify sentinel nodes.
Acknowledgments
None.
Footnote
Conflicts of Interest: JP Ruurda, R van Hillegersberg are both proctors for Intuitive Surgical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Alderson D, Cunningham D, Nankivell M, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol 2017;18:1249-60. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Luketich JD, Nguyen NT, Schauer PR. Laparoscopic transhiatal esophagectomy for Barrett's esophagus with high grade dysplasia. JSLS 1998;2:75-7. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Mariette C, Robb WB. Minimally invasive versus open oesophagectomy for oesophageal cancer. Lancet 2012;380:883-author reply 885-6. [Crossref] [PubMed]
- Swisher S, Ajani J, Correa A, et al. Minimally invasive versus open oesophagectomy for oesophageal cancer. Lancet 2012;380:883-author reply 885-6. [Crossref] [PubMed]
- Weijs TJ, Seesing MF, van Rossum PS, et al. Internal and External Validation of a multivariable Model to Define Hospital-Acquired Pneumonia After Esophagectomy. J Gastrointest Surg 2016;20:680-7. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally invasive versus open esophageal resection: Three-year follow-up of the previously reported randomized controlled trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009;64:135-46. [PubMed]
- Deng J, Su Q, Ren Z, et al. Comparison of short-term outcomes between minimally invasive McKeown and Ivor Lewis esophagectomy for esophageal or junctional cancer: a systematic review and meta-analysis. Onco Targets Ther 2018;11:6057-69. [Crossref] [PubMed]
- Mu J, Gao S, Mao Y, et al. Open three-stage transthoracic oesophagectomy versus minimally invasive thoraco-laparoscopic oesophagectomy for oesophageal cancer: protocol for a multicentre prospective, open and parallel, randomised controlled trial. BMJ Open 2015;5:e008328. [Crossref] [PubMed]
- Briez N, Piessen G, Bonnetain F, et al. Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial - the MIRO trial. BMC Cancer 2011;11:310. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo Classification of Surgical Complications. Five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- Avery KN, Metcalfe C, Berrisford R, et al. The feasibility of a randomized controlled trial of esophagectomy for esophageal cancer-the ROMIO(Randomized Oesophagectomy: Minimally Invasive or Open) study: protocol for a randomized controlled trial. Trials 2014;15:200. [Crossref] [PubMed]
- Sihag S, Kosinski AS, Gaissert HA, et al. Minimally invasive versus open esophagectomy for esophageal cancer: a comparison of early surgical outcomes from the society of thoracic surgeons national database. Ann Thorac Surg 2016;101:1281-8; discussion 1288-9. [Crossref] [PubMed]
- Seesing MFJ, Gisbertz SS, Goense L, et al. A propensity score matched analysis of open versus minimally invasive transthoracic esophagectomy in the Netherlands. Ann Surg 2017;266:839-46. [Crossref] [PubMed]
- Mamidanna R, Bottle A, Aylin P, et al. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg 2012;255:197-203. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Ozawa S, et al. Comparison of short-term outcomes between open and minimally invasive esophagectomy for esophageal cancer using a nationwide database in Japan. Ann Surg Oncol 2017;24:1821-7. [Crossref] [PubMed]
- van Workum F, Stenstra MHBC, Berkelmans GHK, et al. Learning curve and associated morbidity of minimally invasive esophagectomy: a retrospective multicenter study. Ann Surg 2019;269:88-94. [Crossref] [PubMed]
- Guo W, Zou YB, Ma Z, et al. One surgeon's learning curve for video-assisted thoracoscopic esophagectomy for esophageal cancer with the patient in lateral position: how many cases are needed to reach competence? Surg Endosc 2013;27:1346-52. [Crossref] [PubMed]
- Ramage L, Deguara J, Davies A, et al. Gastric tube necrosis following minimally invasive oesophagectomy is a learning curve issue. Ann R Coll Surg Engl 2013;95:329-34. [Crossref] [PubMed]
- Kernstine KH, DeArmond DT, Karimi M, et al. The robotic, 2-stage, 3-field esophagolymphadenectomy. J Thorac Cardiovasc Surg 2004;127:1847-9. [Crossref] [PubMed]
- van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2006;20:1435-9. [Crossref] [PubMed]
- Grimminger PP, Hadzijusufovic E, Babic B, et al. Innovative fully robotic 4-arm Ivor Lewis esophagectomy for esophageal cancer (RAMIE4). Dis Esophagus 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Zhang Y, Han Y, Gan Q, et al. Early outcomes of robot-assisted versus thoracoscopic-assisted Ivor Lewis esophagectomy for esophageal cancer: a propensity score-matched study. Ann Surg Oncol 2019;26:1284-91. [Crossref] [PubMed]
- Park S, Hyun K, Lee HJ, et al. A study of the learning curve for robotic oesophagectomy for oesophageal cancer. Eur J Cardiothorac Surg 2018;53:862-70. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Goldman DA, et al. Early Quality of Life Outcomes After Robotic-Assisted Minimally Invasive and Open Esophagectomy. Ann Thorac Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: A randomized controlled trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Kingma BF, Ruurda JP. Comment on: "Early Outcomes of Robot-Assisted Versus Thoracoscopic-Assisted Ivor Lewis Esophagectomy for Esophageal Cancer: A Propensity Score-Matched Study Ann Surg Oncol 2019;26:1178-81. [Crossref] [PubMed]
- Chao YK. Robotic-assisted esophagectomy vs. video-assisted thoracoscopic esophagectomy (REVATE) trial 2018. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03713749?view=record
- Chao YK, Wen YW, Chuang WY, et al. Transition from video-assisted thoracoscopic to robotic esophagectomy: a single surgeon's experience. Dis Esophagus 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Cuesta MA, Weijs TJ, Bleys RL, et al. A new concept of the anatomy of the thoracic oesophagus: the meso-oesophagus. Observational study during thoracoscopic esophagectomy. Surg Endosc 2015;29:2576-82. [Crossref] [PubMed]
- Weijs TJ, Ruurda JP, Luyer MDP, et al. New insights into the surgical anatomy of the esophagus. J Thorac Dis 2017;9:S675-80. [Crossref] [PubMed]
- Defize IL, Schurink B, Weijs TJ, et al. The anatomy of the thoracic duct at the level of the diaphragm: A cadaver study. Ann Anat 2018;217:47-53. [Crossref] [PubMed]
- Weijs TJ, Ruurda JP, Luyer MDP, et al. Preserving the pulmonary vagus nerve branches during thoracoscopic esophagectomy. Surg Endosc 2016;30:3816-22. [Crossref] [PubMed]
- Weijs TJ, Ruurda JP, Luyer MD, et al. Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy. J Anat 2015;227:431-9. [Crossref] [PubMed]
- Fujita H, Hawahara H, Yamana H, et al. Mediastinal lymph node dissection procedure during esophageal cancer operation-carefully considered for preserving respiratory function. Jpn J Surg 1988;18:31-34. [Crossref] [PubMed]
- van der Horst S, Weijs T, Ruurda JP, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy for esophageal cancer in the upper mediastinum J Thorac Dis 2017;9:S834-42. [Crossref] [PubMed]
- Parry K, Haverkamp L, Bruijnen RC, et al. Surgical treatment of Adenocarcinomas of the Gastro-esophageal Junction. Ann Surg Oncol 2015;22:597-603. [Crossref] [PubMed]
- Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a meta-analysis. Cancer 2006;106:4. [Crossref] [PubMed]
- Kitagawa Y, Kitajima M. Gastrointestinal cancer and sentinel node navigation surgery. J Surg Oncol 2002;79:188-93. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546-53. [Crossref] [PubMed]
- Bhat MA, Naikoo ZA, Dass TA, et al. Role of intraoperative sentinel lymph node mapping in the management of carcinoma of the esophagus. Saudi J Gastroenterol 2010;16:168-73. [Crossref] [PubMed]
- Grotenhuis BA, Wijnhoven BP, van Marion R, et al. The sentinel node concept in adenocarcinomas of the distal esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg 2009;138:608-12. [Crossref] [PubMed]
- Thompson SK, Bartholomeusz D, Jamieson GG. Sentinel Lymph Node Biopsy in Esophageal Cancer: Should It Be Standard of Care? J Gastrointest Surg 2011;15:1762-8. [Crossref] [PubMed]
- Boone J, Hobbelink MGG, Schipper MEI, et al. Sentinel node biopsy during thoracolaparoscopic esophagectomy for advanced esophageal cancer World J Surg Oncol 2016;14:117. [Crossref] [PubMed]
- SNAP study. Available online: https://www.trialregister.nl/trial/5113
- van Hillegersberg R, Seesing MF, Brenkman HJ, et al. Robot-assisted minimally invasive esophagectomy. German version. Chirurg 2016;87:635-42. [Crossref] [PubMed]
- van Rossum PSN, Mohammad NH, Vleggaar FP, et al. Treatment for unresectable or metastatic oesophageal cancer: current evidence and trends. Nat Rev Gastroenterol Hepatol 2018;15:235-49. [Crossref] [PubMed]
- VER. Available online: https://www.verbsurgical.com
- CMR. Available online: https://cmrsurgical.com.
- Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future Stroke Vasc Neurol 2017;2:230-43. [Crossref] [PubMed]
- Marescaux J, Leroy J, Rubino F, et al. Transcontinental robot-assisted remote telesurgery: feasibility and potential applications. Ann Surg 2002;235:487-92. [Crossref] [PubMed]
- Fujiwara H, Shiozaki A, Konishi H, et al. Perioperative outcomes of single‐port mediastinoscope‐assisted transhiatal esophagectomy for thoracic esophageal cancer. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Fujiwara H, Shiozaki A, Konishi H, et al. Transmediastinal approach for esophageal cancer: A new trend toward radical surgery Asian J Endosc Surg 2019;12:30-6. [Crossref] [PubMed]
- Chiu PWY, Ng SSM, Au SKW. Transcervical minimally invasive esophagectomy using da Vinci® SP™ Surgical System: a feasibility study in cadaveric model. Surg Endosc 2019;33:1683-6. [Crossref] [PubMed]
- Hu W, Yuan Y, Chen L. Single-Port Thoracoscopic Minimally Invasive Esophagectomy for Esophageal Cancer. World J Surg 2019;43:567-70. [Crossref] [PubMed]
- Lee JM, Yang SM, Yang PW, et al. Single-incision laparo-thoracoscopic minimally invasive oesophagectomy to treat oesophageal cancer. Eur J Cardiothorac Surg 2016;49:i59-63. [PubMed]
- Lv W, Zeng G, Wu W, et al. Application of single-port video-assisted thoracoscope in treating thoracic oesophageal squamous cell carcinoma using McKeown approach. J Minim Access Surg 2018;14:105-10. [Crossref] [PubMed]