
Aortic dissection patients mimic acute coronary syndrome with preoperative antiplatelet therapy
Introduction
Acute coronary syndrome (ACS) and acute Stanford type A aortic dissection (ATAAD) are the most common diseases characterized by acute chest pain, and both diseases have a high mortality rate. ACS requires emergency treatment to restore coronary flow, so a loading dose of antiplatelet therapy (APT) is given once diagnosed. However, in ATAAD, the principle of treatment is to operate upon diagnosis without delay. In the emergency room, the initial diagnosis of acute chest pain is often ACS. A misdiagnosed case of ATAAD is treated with oral APT, which increases the risk of bleeding, thus affecting the decision-making for surgery. Hence, our center summarized the postoperative outcomes of misdiagnosed ATAAD patients in regard to surgical timing.
Methods
From January 2011 to December 2015, 309 patients with ATAAD received emergency surgery at our center. Of these, 15 patients took oral antiplatelet agents because of a misdiagnosis of ACS before surgery.
The current study was approved by the institutional review board of Nanjing Drum Tower Hospital (No. BL2014004). The requirement to obtain informed consent from the patient was waived, and all authors had full control of the data and information in this study.
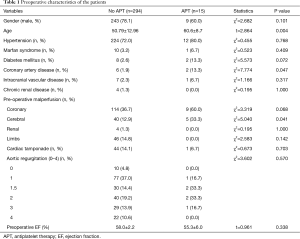
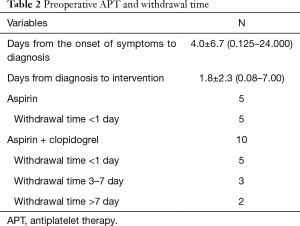
We divided all patients into groups, the APT group with oral antiplatelet agents, and the non-APT group. There were 9 males and 6 females with an overall mean age of 60.6±8.7 years. Twelve patients (80%) had hypertension, and 1 patient was diagnosed with Marfan syndrome. Two patients had a history of coronary heart disease. Thirteen patients had a history of chest pain, 9 patients had myocardial ischemia, and another 2 patients had an acute myocardial infarction (Table 1). All 15 patients received oral APT, 5 of whom took aspirin and discontinued less than 1 day before surgery, and 10 of whom took aspirin and clopidogrel dual APT. Two patients discontinued for 7 days before surgery, 3 patients discontinued for 3 days, and 5 patients underwent emergency surgery for less than 1 day (Table 2).
Full table
Full table
All patients underwent standard surgical procedures. Through computed tomography (CT) scan and intraoperative exploration confirmation, 4 patients who were found with only a primary intimal tear in the ascending aorta and without involvement of the arch received ascending aortic replacement and hemi arch replacement; another 11 patients with different degrees of aortic arch involvement received ascending aortic and total arch replacement with descending aortic stent graft implantation.
The procedure in detail described below. Cannulation through the right axillary artery was completed with or without unilateral femoral artery cannulation; the right atrium was cannulated with the venous cannula, the left ventricular vent cannula was inserted through the right superior pulmonary vein, and retrograde perfusion cannula was inserted through the coronary sinus. The circulatory arrest was instituted after the nasopharyngeal temperature reached 20–22 °C, and unilateral selective cerebral perfusion was performed through the right axillary artery; the flow rate was 3–5 mL/kg per minute. The hemiarch replacement consists of transecting the aorta just before the start of the innominate artery, reinforcing the distal end with a “sandwich” reconstruction and then anastomosing end-to-end with a graft vessel. The total arch replacement should transect the aortic arch between the left common carotid artery and the left subclavian artery, and transect the branches at the root of the three branches. A running suture of 4–0 polypropylene was sewn up the left subclavian artery root stump. A stented elephant trunk was then implanted into the distal descending aorta (Microport, Shanghai, China), then a four-branch graft vessel (Terumo Co., Japan) was attached to the proximal end of the stented graft and distal end of the autologous aortic wall with an end-to-end running suture of 3–0 polypropylene. After the anastomosis was completed the cardiopulmonary bypass (CPB) resumed to normal flow, followed sequentially by anastomosis of the left common carotid artery, left subclavian artery, and the innominate artery. The proximal root underwent Bentall procedure or root reinforcement with the “sandwich” technique, and the ascending aorta was then replaced. After anastomosis of the left common carotid artery, the rewarming began, and the cerebral perfusion was completely restored. Intraoperative myocardial protection was performed by antegrade perfusion of the coronary artery plus retrograde perfusion from the coronary sinus.
Among the 15 patients, 3 had combined coronary artery bypass grafting (CABG), 2 had combined radiofrequency ablation of atrial fibrillation, 1 had combined mitral valve replacement, 1 had combined aortic valve replacement, and 1 had combined aortic valve resuspension.
We used the t-test for continuous variables and presented as mean values with standard deviation (SD). χ2 tests for categorical variables were calculated with Fisher’s exact probability test when necessary and were presented as all numbers with percentage. Wilcoxon rank-sum test was used for continuous non-parametric variables and presented as mean values with the median. A two-tailed P value <0.05 was considered statistically significant.
Results
Preoperative data were compared between patients who did or did not take APT (Table 1). The mean age in the APT group was older than that in the non-APT group (60.6±8.7 vs. 50.79±12.96, P=0.004). The status of preoperative malperfusion seemed worse in the APT group, especially cardiac malperfusion (60.0% vs. 36.7%, P=0.068) and cerebral malperfusion (33.3% vs. 12.9%, P=0.041).
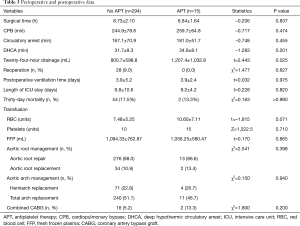
The mean CPB time, cross-clamping time, and deep hypothermic circulatory arrest (DHCA) time were 259.7±64.8, 181.0±51.7, and 34.9±8.1 min, respectively. The differences in operative characteristics were not significant when compared with patients who did not take APT (Table 3). The mean postoperative ventilator duration was 3.9±2.4 days, and the mean intensive care unit (ICU) length of stay was 9.2±4.2 days. Two patients (13.3%) died within 30 days after the operation. The cause of death was a cardiogenic shock in one case, and abdominal aortic dissection ruptured in another. Postoperative outcomes were not associated with whether or not patients took APT (Table 3).
Full table
The blood transfusion measures in all 15 patients in the APT group were as follows: mean red blood cell (RBC) suspension, 10.60±7.11 units; median platelets, 15 units; mean plasma, 1,056.25±580.47 mL. The APT group received more volume of blood transfusion, especially RBC (7.48±5.25 vs. 10.60±7.11 units, P=0.071) and platelet (10 vs. 15 units, P=0.710), but no significant difference was found. Mean 24-h post-operative drainage was 1,207.4±1,032.8 mL in the APT group, which was more than that of patients in the non-APT group (800.7±598.8 vs. 1,207.4±1,032.8 mL, P=0.025) (Table 3).
Ten patients had a preoperative withdrawal of APT time of fewer than 3 days, and 5 patients had a preoperative withdrawal time of more than 3 days. By comparing these two groups, we discovered that patients who had a withdrawal of APT for more than 3 days had a lower mean postoperative drainage than those who had a withdraw of less than 3 days, but the results were not statistically different (1,048.0±375.4 vs. 677.0±139.2 mL, P=0.0997). There was no difference in blood product used and mortality between the two subgroups (Table 4).

Full table
During a mean follow-up of 20.6±17.4 (range, 3–58) months of the 13 patients, 1 patient died suddenly in the 12th month after surgery, and the cause of death was unknown. The remaining patients did not undergo reoperation.
Discussion
Both ATAAD and ACS are characterized by acute chest pain, and the incidence of ACS is 200 times that of aortic dissection (AoD). Therefore, acute chest pain is often diagnosed with ACS as the first diagnosis (1,2). With the improvement of the diagnosis and treatment capacity of primary hospitals, enhanced CT examination of patients with acute chest pain has improved the detection rate of AoD, but some patients still take APT before the diagnosis is clear. The diagnosis of ACS requires an electrocardiogram and cardiac markers. Patients in this study presented with chest pain and chest tightness as the main clinical manifestation. The electrocardiogram showed myocardial ischemia and myocardial infarction, but the cardiac markers did not increase dynamically. They were not absolute indications for taking antiplatelet drugs (3). Therefore, improving the diagnosis and treatment capabilities of the emergency department between ACS and ATAAD is the key to avoiding misdiagnosis. We have used echocardiography (both transthoracic and transesophageal) as a first-line method for acute dissection patients: we can identify the intimal tears, the involvement of dissection, contraction disturbance of the left ventricle, and cardiac tamponade. Because of the convenience and effectiveness of echocardiography, we suggest that bedside echocardiography should be the necessary check method for acute chest pain patients in order to distinguish ATAAD from ACS cases (4,5).
APT increases the risk of perioperative bleeding in cardiac surgery and the number of blood products used, which in turn increases the risk of perioperative death. The guidelines indicate that most patients do not need to stop aspirin before surgery and that dual APT is used in low bleeding risk patients, but patients with high-risk bleeding need to withdraw for 5–7 days before surgery (6). Patients in this study taking aspirin alone or aspirin and clopidogrel had significantly increased postoperative drainage and usage of intraoperative blood product, but the mortality rate did not increase significantly. There were no significant differences in drainage, usage of blood products, and mortality between patients who discontinued antiplatelet agents for less than 3 days compared with patients who discontinued the drugs for more than 3 days. Some scholars have reported that in ATAAD, coagulation function is impaired, and is associated with preoperative abnormal activation of coagulation function and intraoperative hypothermic circulatory arrest. Therefore, ATAAD itself causes coagulation abnormalities (7), and, although oral APT significantly affected platelet function, there was no significant increase in bleeding and mortality risk compared with the non-administration of APT.
The preferred treatment for ATAAD patients is surgical correction, and ATAAD patients have indications for emergency surgery. Studies have shown that the later the surgical intervention, the higher the mortality rate (8). At the same time, the longer the onset time, the more stable the natural history of the patient is. For patients with a definite diagnosis of aortic dissection for more than 7 days, stopping APT for a reasonable amount of time before surgery can be considered. In this study, 2 patients underwent surgery 7 days after discontinuation of the antiplatelet drugs. These 2 patients were diagnosed 7 days since onset, and aortic rupture or other related complications did not occur during the wait. Therefore, the timing of emergency surgery for this type of patient needs to take into account both the withdrawal time of the antiplatelet drug and the risk of rupture of the dissection.
Compared with conventional cardiac surgery, patients in this study received a larger number of blood products, and in patients taking antiplatelet drugs, the transfusion of platelets has a significant effect on increasing platelet count and function (9). Thromboelastogram (TEG) can clarify platelet function and guide the perioperative coagulation function evaluation and application of blood products. Meanwhile, the life-threatening condition of ATAAD limits the clinical conduct of preoperative TEG and other blood tests. At present, we routinely use TEG to evaluate the preoperative coagulation function of patients with dissection, which aids in the analysis.
This study is a single-center, retrospective study with a small sample size which may limit the generalizability of the clinical characteristics of such patients. Nonetheless, the clinical findings have helped clarify how the misdiagnosis of ATAAD as ACS can be avoided, and have shown that surgical outcomes can be safe even with more blood loss and transfusion.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (Nos. 81670437). No other funding can be connected to any of the authors.
Footnote
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The current study was approved by the institutional review board of Nanjing Drum Tower Hospital (No. BL2014004). The requirement to obtain informed consent from the patient was waived, and all authors had full control of the data and information in this study.
References
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Fox KA, Eagle KA, Gore JM, et al. The global registry of acute coronary events, 1999 to 2009--Grace. Heart 2010;96:1095-101. [Crossref] [PubMed]
- Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999-3054. [Crossref] [PubMed]
- Evangelista A, Flachskampf FA, Erbel R, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr 2010;11:645-58. [Crossref] [PubMed]
- Sobczyk D, Nycz K. Feasibility and accuracy of bedside transthoracic echocardiography in diagnosis of acute proximal aortic dissection. Cardiovasc Ultrasound 2015;13:15. [Crossref] [PubMed]
- Rossini R, Musumeci G, Visconti LO, et al. Perioperative management of antiplatelet therapy in patients with coronary stents undergoing cardiac and non-cardiac surgery: a consensus document from Italian cardiological, surgical and anaesthesiological societies. EuroIntervention 2014;10:38-46. [Crossref] [PubMed]
- Guan XL, Wang XL, Liu YY, et al. Changes in the hemostatic system of patients with acute aortic dissection undergoing aortic arch surgery. Ann Thorac Surg 2016;101:945-51. [Crossref] [PubMed]
- Booher AM, Isselbacher EM, Nienaber CA, et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med 2013;126:730.e19-24. [Crossref] [PubMed]
- Thiele T, Sümnig A, Hron G, et al. Platelet transfusion for reversal of dual antiplatelet therapy in patients requiring urgent surgery: a pilot study. J Thromb Haemost 2012;10:968-71. [Crossref] [PubMed]


