The comparison of ultrasound-guided thoracic paravertebral blockade and internal intercostal nerve block for non-intubated video-assisted thoracic surgery
Introduction
Non-intubated thoracic anesthesia is not an innovative technique. During the period of 1940–1950, hundreds of major thoracic surgery procedures, including major lung resections and esophagectomies, have been successfully performed using local anesthesia techniques in awake patients (1). Subsequently, the development of double-lumen tube ventilation by Bjork and Carlens in 1950s (2) led to the birth of modern thoracic surgery. The use of general anesthesia (GA) and single-lung ventilation rapidly became the gold-standard.
With the rapid development of enhanced recovery protocols and cost-containment, the past experience of awake thoracic surgery has been re-examined with the increasing understanding of the complications of GA for tracheal intubation and mechanical ventilation. As a result, the concept of non-intubated thoracic surgery is gaining renewed attention. In 2004, Pompeo first reported a case of video-assisted thoracic surgery (VATS) wedge resection of non-intubated anesthesia with spontaneous breathing (3). Subsequently, a growing number of thoracic surgical procedures using non-intubated thoracic anesthesia techniques have been reported.
The anesthetization protocol of NIVATS combines moderate sedation with local anesthesia, with the latter being the more crucial aspect. Although thoracic epidural anesthesia (TEA) is considered to be the gold standard of postoperative analgesia in VATS, it has many complications, including dural puncture, neurological injury, and paraplegia (4). Therefore, thoracic paravertebral blockade (TPVB) and intercostal nerve block (ICNB) are effective alternatives with fewer contra-indications, having been successfully used in NIVATS (5,6). The team of National Taiwan University Hospital summarized the safe and effective use of internal intercostal nerve block (IINB) and vagal block in NIVATS (5). However, this method requires opening a small observation port in the chest to perform internal intercostals block. We hypothesized that the establishment of local anesthesia such as TPVB before thoracotomy would reduce the use of intravenous anesthesia and better preserve the patient’s spontaneous breathing. Consequently, the aim of this study was to analyze and compare the perioperative results of the two methods in NIVATS.
Methods
Subjects
We retrospectively collected the clinical information of 34 consecutive patients who underwent non-intubated thoracoscopic surgery with specialized anesthetization from April 2016 to May 2017. Twenty patients received TPVB (P group), and the other 14 received IINB (I group). The exclusion criteria were the following: the American Society of Anesthesiologists (ASA) physical status classification greater than 3; body mass index (BMI) >30 kg/m2; and presence of coagulopathy, neurological disorders, or other anatomical abnormalities. The study was approved by the Institutional Ethics Committee and informed consent was obtained from all patients.
Preoperative procedures
On arrival in the operating room, the vein access of the upper limbs was first established. Electrocardiography (ECG), blood pressure (BP), pulse oxygen saturation (SpO2), respiratory rate (RR), and bispectral index (BIS) of the patient were monitored. Anesthesia was induced using 0.1 µg/kg of sufentanil, along with a target-controlled infusion (TCI) of propofol with a target effective-site concentration (Ce) of 3–4 µg/mL via a TCI infuser pump (Fresenius Kabi). When the BIS index was less than 50, LMA (laryngeal-mask airway; Royal Fornia Medical Equipment Co., Ltd, China) was inserted into the patient. Using synchronized intermittent mandatory ventilation (SIMV) mode for ventilation, the tidal volume, frequency and p-support were maintained at 6–8 mL/kg, 15 bpm and 10–13 mmHg respectively. The SIMV was stopped at the beginning of the operation, and the patient was induced to resume spontaneous breathing. The radial artery was catheterized for continuous invasive BP. When the operation was expected to exceed 3 hours, a central venous catheter was inserted into the right internal jugular vein. After completing the above procedures, the patient was placed in a lateral position.
Procedure of paravertebral block
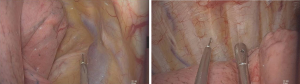
Paravertebral blockade was performed between the thoracic segments T4-5 and T7-8, using ultrasound guidance. A linear ultrasound transducer (HITACHI Arietta 60) was placed intercostally to identify the thoracic paravertebral space (TPVS), and a 20-gauge needle was inserted into the plane of the transducer. When the needle tip reached TPVS, 10 mL 0.375% ropivacaine was injected at each level, making the total amount 20 mL.
Procedure of IINB
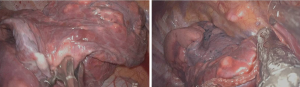
After local infiltration with 2% lidocaine, an observation port, operation port and an auxiliary port were made. Under thoracoscopic guidance, IINB was induced with 1% lidocaine and 0.375% ropivacaine mixture (1.5 mL for each intercostal space) from the 3rd to the 8th intercostal nerve under the parietal pleura, 2 cm lateral to the sympathetic chain using a 25-G infusion needle (Figure 1).
Surgery
The surgical procedure was the same in both groups. Surgical techniques for thoracoscopic wedge resection, lobectomy, and bullae resection have been described previously (7,8). Briefly, a three-port method was used to perform thoracoscopic surgery, and the observation port was located between the 6th and 8th intercostal. The auxiliary port and the operation port were located at the same area between the 4th and 5th intercostal cartilages. The observation port marked the starting point for opening the chest. A 1-cm 30° thoracoscope was used for visualization, and under direct thoracoscopic vision, 5 mL of 1% lidocaine and 0.375% ropivacaine mixture was injected into the trunk surrounding the vagus nerve (Figure 1). After the surgery, the pleural cavity was closed, the wound was sutured, and the intravenous anesthetic was stopped. The patient was sent to the post-anesthesia care unit (PACU). When the patient was conscious and the deglutition reflex was restored, the LMA was removed. One hour after the patient showed full recovery from the anesthesia, the visual analog scale (VAS) (scores 0= no pain to 10= unbearable pain) was used to assess the degree of pain.
Data collection
The blood-gas analysis was performed 15 minutes after opening the chest. The consumption of propofol and remifentanil from the beginning of anesthesia induction to 15 minutes after opening the chest was recorded.
Statistical analysis
To minimize the effect of selection bias, we performed Propensity Score Matching (PSM) for clinical characteristics. The propensity score was calculated using a logistic regression analysis with the dependent variable being the type of imaging procedure and the independent covariates being the following baseline clinical and demographic variables: age, gender, BMI, and types of thoracoscopic operation.
A 1:1 nearest neighbor matching algorithm that pairs patients with the closest propensity scores was used. A caliper width of 0.2 units was used. The procedure of propensity scores yielded 2 matched cohorts of 14 patients. We compared standardized differences for all covariates between prematch and postmatch.
Continuous variables were presented as mean ± standard deviation and compared using the independent samples t- test. Categorical variables were presented as numbers and were compared by Chi-square test or Fisher’s exact test. All data were analyzed with SPSS software (SPSS version 25.0; IBM Corp., Armonk, NY, USA). P values <0.05 were considered statistically significant.
Results
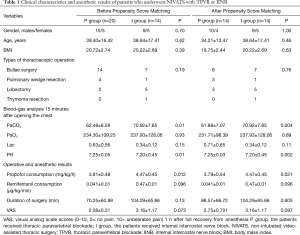
The clinical characteristics of the two matched groups extracted by propensity analysis are presented in Table 1. There were no significant differences between the two groups in terms of gender, age and BMI (P>0.05). The types of thoracoscopic operations that were performed, including bullae surgery, pulmonary wedge resection, lobectomy, and thymoma resection are summarized in Table 1, and were not significantly different between the P and I groups (P>0.05).
Full table
Blood-gas analysis of 15 minutes after opening the chest is shown in Table 1. The PaCO2 in the P group was significantly lower than the I group (P=0.004). The operative and anesthetic results are summarized in Table 1. The consumption of propofol from the beginning of anesthesia induction to 15 minutes after opening the chest in P group was also significantly less compared to the I group (P=0.021). There were no significant differences between the two groups in terms of surgery duration and VAS scores. None of the cases involved switching to one lung ventilation with endotracheal intubation.
Discussion
Multiple studies in the last decade have shown the advantages of using non-intubated video-assisted thoracic surgery (NIVATS) over GA with endotracheal intubation, including avoiding intubation related airway injury and one lung ventilation induced lung injury, as well as obviating the effect of residual neuromuscular block (9-12). A few studies have shown that postoperative recovery may be better in patients with NIVATS than in patients with GA with endotracheal intubation (8,12,13).
According to previous studies and our experience, the physiological changes after NIVATS thoracotomy: following opening of the thorax, atmospheric air enters into the chest cavity, the lung on the surgical side will gradually collapse (Figure 2). However, if the patient has an apnea, or a glottic closure caused by pain, this may result in the lungs on the surgical side cannot collapsing. Therefore, it is important to preserve the patient's spontaneous breathing during the NIVATS process to ensure the operation space.
To the best of our knowledge, local wound infiltration, selective ICNB, TPVB and TEA are some of the anesthetic techniques that can be considered for NIVATS (12). TEA is the gold standard of VATS anesthesia as it can provide a longer analgesic effect. However, it has various complications such as dural puncture, neurological injury and paraplegia that should be considered (14).
Hung et al. successfully used internal ICNB, vagal block, and targeted sedation in the NIVATS of 109 patients (5). Furthermore, they proved that multiple ICNBs exerted an anesthetic-sparing effect in the NIVATS (15). The advantages of this method include its accuracy and reliability under the guidance of thoracoscopy. However, we found that it was necessary to use intravenous and local wound anesthesia to prevent pain and body movements before inducing IINB. The dose of intravenous anesthesia should be regulated as an overdose can inhibit the patient's breathing. Furthermore, the concentration of local anesthetics at the site of surgical incision should also be controlled since excessive amounts can leave too much fluid in the incision, which will affect the operation of the electric knife. In 2010, Piccioni et al. first reported the successful use of thoracic PVB in the VATS of two cancer patients (6). However, no previous study has compared PVB and IINB for NIVATS. We hypothesized that pre-established local anesthesia could better maintain the patient’s spontaneous breathing than perform peripheral nerve block after entering the thoracic cavity. To investigate this hypothesis, we retrospectively analyzed 34 patients who received either TPVB or IINB before NIVATS.
The procedure uses regional anesthesia to prevent incisional pain and cough reflex, and intravenous anesthesia to keep the patient sedated. However, the excessive use of anesthetic drugs can cause respiratory depression (16,17). In addition, surgical pneumothorax, respiration of one lung on the non-surgical side, and intravenous anesthetization, can result in retention of carbon dioxide. Also, hypercapnia may cause deep breathing and a large shift of the mediastinum, consequently impacting surgery. Furthermore, severe hypercapnia may lead to intracranial hypertension and myocardial depression. Therefore, optimal regional anesthesia is an important part of NIVATS for maintaining spontaneous respiration during the operation, and avoiding severe hypercapnia.
In this study, the PaCO2 of the P group was significantly lower than that of the I group 15 minutes after the opening of the chest cavity, owing to the lower amount of propofol used in the P group. Furthermore, since the patients in the P group had obtained a sufficient anesthetic effect on the chest, the anesthetist was not concerned about the movement and awareness of the patient at the time of skin incision and injection of local anesthetic.
Our results showed that ultrasound-guided TPVB can provide a sufficient and accurate analgesic effect on the chest wall with minimal complications, which can accordingly lessen the use of anesthetics at the beginning of the surgery (skin incision and insertion into the first thoracic trocar). Lower anesthetic use would in turn better maintain spontaneous breathing.
According to a meta-analysis by Davies et al. (4), although TPVB and TEA provide comparable pain relief after thoracic surgery, TPVB has fewer side-effects. In this study, ultrasound-guided thoracic paravertebral nerve block was used. TPVB can be performed using anatomical landmarks or ultrasound guided techniques. Ultrasound-guided TPVB can improve the success rate, shorten the puncture time, and reduce complications of pneumothorax and vascular puncture (18). In 2009, Ben-Ari and Shibata described using an intercostal approach in ultrasound guided TPVB (19,20). This study is based on this puncture technique. Among the patients in this study, there were no complications such as local anesthetic poisoning and pneumothorax, which confirmed that this method can be safely and effectively used in NIVATS.
There are certain limitations to our study. First, it was a retrospective study with a small sample size and the findings therefore have to be confirmed with a larger RCT. Second, since a single dose of TPVB can provide sufficient pain relief, inserting a catheter into the paravertebral space for continuous infusion of local anesthetics may provide protracted post-surgery analgesia. Furthermore, different doses and drugs between TPVB and IINB may cause bias of the results. More studies have to be conducted to clarify this question.
In conclusion, ultrasound-guided TPVB can provide safe and reliable local anesthesia for NIVAT by reducing the effective dosage of intravenous anesthetics and more effectively maintaining spontaneous breathing.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University [Medical research ethics review (2019)No.K-27] and informed consent was obtained from all patients.
References
- Pompeo E, Mineo TC. Thoracic surgery: a historical perspective. In: Pompeo E. editor. Awake thoracic surgery. Bentham Books, 2012:3-8.
- Bjork VO, Carlens E. The prevention of spread during pulmonary resection by the use of a double-lumen catheter. J Thorac Surg 1950;20:151-7. [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418-26. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [Crossref] [PubMed]
- Piccioni F, Langer M, Fumagalli L, et al. Thoracic paravertebral anaesthesia for awake video-assisted thoracoscopic surgery daily. Anaesthesia 2010;65:1221-4. [Crossref] [PubMed]
- Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis 2012;4:126-30. [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Kiss G, Claret A, Desbordes J, et al. Thoracic epidural anaesthesia for awake thoracic surgery in severely dyspnoeic patients excluded from general anaesthesia. Interact Cardiovasc Thorac Surg 2014;19:816-23. [Crossref] [PubMed]
- Miñambres E, Buron J, Ballesteros MA, et al. Tracheal rupture after endotracheal intubation: a literature systematic review. Eur J Cardiothorac Surg 2009;35:1056-62. [Crossref] [PubMed]
- Murphy GS, Szokol JW, Avram MJ, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg 2013;117:133-41. [Crossref] [PubMed]
- Kiss G, Castillo M. Non-intubated anesthesia in thoracic surgery-technical issues. Ann Transl Med 2015;3:109. [PubMed]
- Tacconi F, Pompeo E. Non-intubated video-assisted thoracic surgery: where does evidence stand? J Thorac Dis 2016;8:S364-75. [Crossref] [PubMed]
- D'Ercole F, Arora H, Kumar PA. Paravertebral Block for Thoracic Surgery. J Cardiothorac Vasc Anesth 2018;32:915-27. [Crossref] [PubMed]
- Wang ML, Hung MH, Chan KC, et al. Intraoperative multiple intercostal nerve blocks exert anesthetic-sparing effect: A retrospective study on the effect-site concentration of propofol infusion in nonintubated thoracoscopic surgery. Acta Anaesthesiol Taiwan 2016;54:77-80. [Crossref] [PubMed]
- Carmi U, Kramer MR, Zemtzov D, et al. Propofol safety in bronchoscopy: prospective randomized trial using transcutaneous carbon dioxide tension monitoring. Respiration 2011;82:515-21. [Crossref] [PubMed]
- Heuss LT, Schnieper P, Drewe J, et al. Safety of propofol for conscious sedation during endoscopic procedures in high-risk patients-a prospective, controlled study. Am J Gastroenterol 2003;98:1751-7. [PubMed]
- Pace MM, Sharma B, Anderson-Dam J, et al. Ultrasound-Guided Thoracic Paravertebral Blockade: A Retrospective Study of the Incidence of Complications. Anesth Analg 2016;122:1186-91. [Crossref] [PubMed]
- Ben-Ari A, Moreno M, Chelly JE, et al. Ultrasound-guided paravertebral block using an intercostal approach. Anesth Analg 2009;109:1691-4. [Crossref] [PubMed]
- Shibata Y, Nishiwaki K. Ultrasound-guided intercostal approach to thoracic paravertebral block. Anesth Analg 2009;109:996-7. [Crossref] [PubMed]