
Dynamic magnetic resonance imaging in unilateral diaphragm eventration: knowledge improvement before and after plication
Introduction
The assessment of diaphragm eventration before surgical plication is not clearly defined and no precise criteria exist to really understand which patient is operated with which results depending on the technique used. In fact, the basic assessment includes standard chest X-ray—end-inspiration and end-expiration—sniff-test during fluoroscopy (1,2), computed tomography (CT) and sometimes ultrasonography (3,4). Dynamic magnetic resonance imaging (dMRI) has been described as a new method of functional diaphragmatic imaging, allowing an exploration of the complex shape and the motions of the diaphragm (5), but it remains underused (6).
The objective of this study was to determine measurement criteria using dMRI to more precisely assess the preoperative and postoperative functional characteristics of each hemidiaphragm (HD) in patients treated for unilateral HD eventration.
Methods
Between 2006 and 2017, 40 patients were referred to our centre for HD eventration. Because of incapacitating symptoms, 36 patients underwent surgical treatment. Among them, the last 18 operated patients (patient group: Gp1) had dMRI of the diaphragm in addition to the usual assessment by clinical evaluation, chest CT scan and pulmonary function tests (PFT). All patients gave their written informed consent for analysis of their data and the study was approved by the Ethical Committee of the French Society of Thoracic and Cardio-Vascular Surgery (CERC-SFCTCV-2018-9-27-20-32-39-LeFr).
Dynamic MRI was performed on a GE 1.5 T machine (Signa, GE Healthcare, Chicago, IL, USA). The dMRI protocol was established by our referring thoracic radiologist, and was first tested in five healthy volunteer subjects representing the control group (group2: Gp2). Subsequently, the protocol was applied to Gp1 patients. The diaphragm analysis was done on coronal and sagittal views, at two periods of breathing (deep inspiration and expiration) by T1 weighted sequences and T2 weighted short sequences (Fiesta one image per second) imaging the whole diaphragmatic area. For the dynamic exploration, each patient was asked to breathe deeply and regularly in order to get a complete respiratory cycle (from forced inspiration to forced expiration) (Figure 1).

The analysed criteria in dMRI
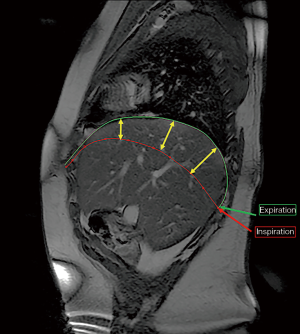
The HD excursion: on both sides [injured HD (IHD), or side of eventration, and healthy HD, or contralateral side] during a single respiratory cycle from end-inspiration to end-expiration. This measurement is done at three fixed sites on sagittal view on dMRI. The three sites were defined using a perpendicular plane to the surface of the HD at 25%, 50% and 75% respectively of the total length of the HD from the front to the back (Figure 2). The mean, lowest and highest values of these measurements in Gp2 were considered as references to compare with Gp1.
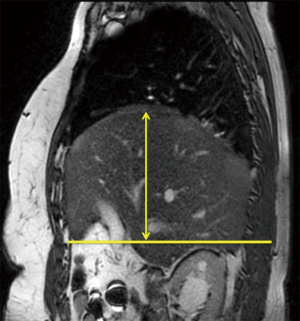
The HD height of curvature: is defined on sagittal planes by measuring the longest radius that crosses a horizontal line issuing from the origin of the xiphoid process. It is a fixed point for each patient (Figure 3). The reduction of this HD height of curvature shows the importance of the surgical tightening.
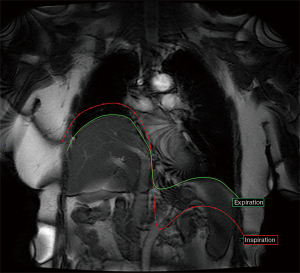
The presence of an upward paradoxical movement of the IHD: at the end of inspiration the healthy side is down and the injured side goes up (Figure 4).
All these criteria were systematically measured by a two-specialist team including a radiologist and a thoracic surgeon, not involved in the patients’ care. The same protocol by dMRI was repeated one to three months after the surgical treatment in available patients. In fact, some patients were living too far away so they were only interviewed by telephone.
Other criteria were evaluated: the patients self-reported their respiratory discomfort using a dyspnoea visual analogue scale (VAS) in the preoperative and postoperative periods. A nurse, independent from the surgical team, collected the results of that evaluation. Pulmonary function tests were done one month before and more than one month after surgery.
The surgical procedure was done through a video-assisted short lateral thoracotomy. In 15 cases we performed the diaphragm plication reinforced by a non-resorbable prosthetic mesh because of major muscular atrophy. In one patient (No. 16) the diaphragm plication was performed alone, and in two patients (No. 2,13) only a prosthetic implant was placed because of an extremely thin HD.
As the physiological diaphragm excursion is not exactly comparable on both sides, we separated Gp1 patients into two groups according to the side of eventration. Gp1L included all patients with an injured left HD (n=15) and Gp1R the patients with an injured right HD (n=3). The clinical characteristics for all patients are summarised in Table 1.
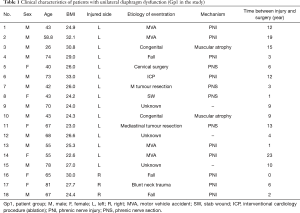
Full table
Statistical analyses
Preoperative and postoperative continuous data were presented as median and interquartile ranges and compared using Wilcoxon signed-rank test. Categorical data were expressed as percentages. The control group (Gp2) and the experimental group (Gp1) were compared with Mann-Whitney test. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS software (SAS Institute, Inc., Cary, NC, USA).
Results
Preoperative evaluation
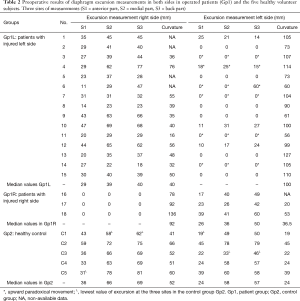
The three excursion measurements in both groups are reported in Table 2. The analysis in Gp2 confirms the physiological front-to-back gradient. In this group, the median and the lowest values of the HD excursion at the three sites were respectively 36, 66, 69 mm and 31, 58, 62 mm on the right side; 24, 58, 57 mm and 19, 33, 46 mm on the left side.
Full table
In Gp1L, ten patients had no excursion of the IHD and four of them even had an upward paradoxical movement (No. 3,7,11,14). In two other patients presenting persistent contractions (No. 4,6), an upward paradoxical movement was observed.
In Gp1R, no contraction or paradoxical movement of the IHD was observed. Compared to values in Gp2, the healthy HD excursions in Gp1L were significantly reduced at S1 (P=0.038), S2 (P=0.006) and S3 (P=0.004) sites. Using multivariate analyses, no difference in age of patients, time of eventration, values of height of curvature, vital capacity or other criteria were identified for explaining this contralateral dysfunction.
Postoperative evaluation
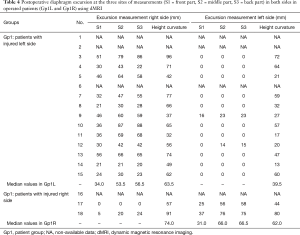
Four patients were not evaluated by dMRI because they had moved away. They only had clinical questioning by telephone. Fourteen available patients had dynamic MRI performed one to three months after surgery (Tables 3,4). The stabilisation of the IHD at a good level was constantly observed and all paradoxical movements disappeared (Figure 5). The postoperative height of curvature of the plicated HD was significantly reduced in all patients in Gp1L who sustained MRI after surgery [median 50 (41, 68.5), P=0.0005]. An increased excursion of the healthy HD was observed in eight patients: six in Gp1L, among the eight who had a preoperative reduction and postoperative dMRI, and two in Gp1R. Finally, we no longer observed any difference in the healthy HD excursion at the three sites and height of curvature in Gp1L compared to control group Gp2.
Full table
Full table

In Gp1R, the two patients (No. 17,18) who did not have any contraction in their IHDs had postoperative dMRI. One patient had contraction recovery associated with front-to-back gradient restoration. In those two cases, we also observed an improvement of the excursion and curvature on the healthy HD up to near-normal values.
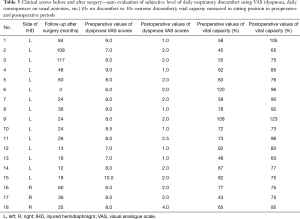
The median follow-up of the patients was 30.5 months (3 to 117 months). Patient No. 6 died 3 months after the operation of a cause not related at all to the diaphragmatic pathology. In all patients dyspnoea VAS scores significantly decreased 3 months after surgery [mean values from 7.9 to 1.8 with median decreasing 6 (5.5,7.0), P<0.0001] and vital capacity increased [mean value from 68.7% to 84% with median improvement 16.5 (10.0,23.0), P=0.002].
Discussion
The criteria we developed to analyse the diaphragm excursion using dMRI—height of curvature and excursion at three sites—allowed us to compare the IHD dysfunction from one patient to another or to healthy subjects, and to follow its evolution over time. These criteria highlighted the existence of a preoperative unknown dysfunction in 81% of the right healthy HDs in Gp1L reversible after surgery. This point has never been demonstrated to date. Only a recent study identified a reduced pressure generated by the healthy HD after phrenic nerve stimulation in patients with contralateral unilateral phrenic nerve paralysis (9).
The cause of the contralateral dysfunction is probably due to a muscular fatigue mechanism induced by increased work to get normal breathing. It certainly encourages surgeons to propose plication of the IHD without too much delay especially when the patient has other comorbidities. In case of a suspected bilateral dysfunction, stimulation tests with diaphragmatic electromyogram are recommended to get more precise information about the phrenic nerves conduction and the muscle strength (10,11) but these exams are not commonly available.
The main difficulty to measure the diaphragm excursion is the vertical apposition area, disappearing at total lung capacity (12) but representing 45%±1.5% of the total diaphragm surface (13).
In our study we used fixed markers on the chest wall and diaphragmatic area, allowing an accurate measurement of the front-to-back excursion with a loss of the physiological gradient (14,15) in 83% of patients.
In 2004, Takazakura et al. analysed the postural differences of diaphragm motions between sitting and supine positions on 10 healthy subjects using dMRI protocol in a vertical open machine (16). Three diaphragmatic points of 6 sagittal planes for both positions were used to measure the diaphragmatic excursions. A significantly greater excursion of the diaphragm in supine position compared to sitting position was found for 15 of the 18 points. In 5 out of the 6 sagittal planes, the front-to-back gradient was significantly higher in supine position than in sitting position. The limits of that study were directly linked to the complex craniocaudal and outward movements of the diaphragm with measures not using fixed points. Takazakura’s study was the first quantitative analysis of diaphragm motions in supine position probably giving the most physiological results for diaphragm excursion measurement (16). However, vertical open machines are not commonly available.
The goal of other studies developing different measurement criteria using dMRI has been to evaluate the lung motions rather than the diaphragm mobility itself, the final goal being high precision radiotherapy for lung tumours (17).
The upward paradoxical movement of the IHD visible on chest X-ray was already described in older series (14). In our study, we identified this abnormal movement only in 33% of left HD eventrations without any link to the time interval after injury. In the literature, the incidence on sniff test (18) or ultrasound (19) is from 0% to 12% or even 100% on either side.
To precisely and separately analyse each HD, only an imaging exam such as dMRI or ultrasound may be proposed. Ultrasound is probably the simplest, most informative and most useful exam (20). However, it does not allow the simultaneous study of both HDs and it is operator-dependent. For more than ten years in our centre we have systematically included dMRI in the assessment of diaphragm eventrations. It is done as soon as the HD injury is suspected or discovered, in order to have an initial evaluation. The identification of very weak but persistent contractions of the whole IHD means that the phrenic nerve is functional with hope of spontaneous recovery. If plication is however decided, identifying such persistent contractions before surgery is important in order to preserve the phrenic nerve during the surgical procedure whatever the surgical technique—video or open surgery, using prosthetic mesh or not. Persistent contractions are probably underestimated, but they were found in 28% of the cases in our series. However, our series is short so we cannot demonstrate that phrenic nerve preservation at time of plication, in case of preoperative persistent contractions in the IHD, allows active HD contraction after surgery.
Limits of the study: dMRI requires the patients’ active participation to precisely measure the excursion of each HD. In case of poor understanding of the breathing manoeuvres, like in three cases in our series, the measurements may be underestimated. Patients with morbid obesity or claustrophobia cannot be inserted inside the closed machine, whose high cost and low availability is also a limiting factor.
Conclusions
Giving a real-time global kinetic view of both HDs, dMRI not only improves the knowledge of unilateral diaphragm eventration but also permits to evaluate the quality of its surgical repair. The analysis grid we developed makes it possible to identify a healthy HD dysfunction, reversible after contralateral plication. Finally, we consider that dMRI should be included in the medical evaluation before any diaphragm plication to compare the different techniques.
Acknowledgments
The authors are grateful to Bill Fry and Valerie Mege Lin for editing English style and grammar.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients gave their written informed consent for analysis of their data and the study was approved by the Ethical Committee of the French Society of Thoracic and Cardio-Vascular Surgery (CERC-SFCTCV-2018-9-27-20-32-39-LeFr).
References
- Demos DS, Berry MF, Backhus LM, et al. Video-assisted thoracoscopic diaphragm plication using a running suture technique is durable and effective. J Thorac Cardiovasc Surg 2017;153:1182-8. [Crossref] [PubMed]
- Verschakelen JA, Deschepper K, Jiang TX, et al. Diaphragmatic displacement measured by fluoroscopy and derived by Respitrace. J Appl Physiol (1985) 1989;67:694-8. [Crossref] [PubMed]
- Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 2013;47:319-29. [Crossref] [PubMed]
- Sferrazza Papa GF, Pellegrino GM, Di Marco F, et al. A review of the ultrasound assessment of diaphragmatic function in clinical practice. Respiration 2016;91:403-11. [Crossref] [PubMed]
- Cai J, Sheng K, Benedict SH, et al. Dynamic MRI of grid-tagged hyperpolarized helium-3 for the assessment of lung motion during breathing. Int J Radiat Oncol Biol Phys 2009;75:276-84. [Crossref] [PubMed]
- Kharma N. Dysfunction of the diaphragm: imaging as a diagnostic tool. Curr Opin Pulm Med 2013;19:394-8. [PubMed]
- Le Pimpec-Barthes F, Hernigou A, Mazzella A, et al. Preoperative dMRI in a patient with left eventration—coronal plane. Asvide 2019;6:248. Available online: http://www.asvide.com/watch/32933
- Le Pimpec-Barthes F, Hernigou A, Mazzella A, et al. Postoperative dMRI in the same patient (Figure 1)—coronal plane after left plication. Asvide 2019;6:249. Available online: http://www.asvide.com/watch/32934
- Caleffi-Pereira M, Pletsch-Assunção R, Cardenas LZ, et al. Unilateral diaphragm paralysis: a dysfunction restricted not just to one hemidiaphragm. BMC Pulm Med 2018;18:126. [Crossref] [PubMed]
- Similowski T, Fleury B, Launois S, et al. Cervical magnetic stimulation: a new painless method for bilateral phrenic nerve stimulation in conscious humans. J Appl Physiol (1985) 1989;67:1311-8. [Crossref] [PubMed]
- Shehu I, Peli E. Phrenic nerve stimulation. Eur J Anaesthesiol Suppl 2008;42:186-91. [Crossref] [PubMed]
- Cluzel P, Similowski T, Chartrand-Lefebvre C, et al. Diaphragm and chest wall: assessment of the inspiratory pump with MR imaging-preliminary observations. Radiology 2000;215:574-83. [Crossref] [PubMed]
- Paiva M, Verbanck S, Estenne M, et al. Mechanical implications of in vivo human diaphragm shape. J Appl Physiol (1985) 1992;72:1407-12. [Crossref] [PubMed]
- Alexander C. Diaphragm movements and the diagnosis of diaphragmatic paralysis. Clin Radiol 1966;17:79-83. [Crossref] [PubMed]
- Simon G, Bonnell J, Kazantzis G, et al. Some radiological observations on the range of movement of the diaphragm. Clin Radiol 1969;20:231-3. [Crossref] [PubMed]
- Takazakura R, Takahashi M, Nitta N, et al. Diaphragmatic motion in the sitting and supine positions: healthy subject study using a vertically open magnetic resonance system. J Magn Reson Imaging 2004;19:605-9. [Crossref] [PubMed]
- Plathow C, Schoebinger M, Fink C, et al. Quantification of lung tumor volume and rotation at 3D dynamic parallel MR imaging with view sharing: preliminary results. Radiology 2006;240:537-45. [Crossref] [PubMed]
- Graham DR, Kaplan D, Evans CC, et al. Diaphragmatic plication for unilateral diaphragmatic paralysis: a 10-year experience. Ann Thorac Surg 1990;49:248-51; discussion 252. [Crossref] [PubMed]
- Higgs SM, Hussain A, Jackson M, et al. Long term results of diaphragmatic plication for unilateral diaphragm paralysis. Eur J Cardiothorac Surg 2002;21:294-7. [Crossref] [PubMed]
- Kim WY, Suh HJ, Hong SB, et al. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 2011;39:2627-30. [Crossref] [PubMed]


