
The tidal volume fix and more…
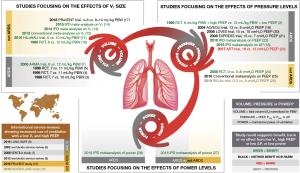
In the past 30 years the medical literature has been enriched with numerous publications reporting on randomized clinical trials, meta-analysis and service reviews on invasive ventilation in critically ill patients (Figure 1). For many years the investigations on lung-protective ventilation focused on patients with acute respiratory distress syndrome (ARDS). Interest in the effects of lung-protective ventilation in patients without ARDS is rapidly increasing, however, resulting in a steep rise of the number of publications on lung-protection in patients without ARDS.
The seminal ARMA trial performed by the ARDS network-investigators two decades ago convincingly showed survival benefit from a ventilation strategy with a low tidal volume (VT) [6 mL/kg predicted body weight (PBW]] when compared to a ventilation strategy with a high VT (12 mL/kg PBW) in patients with ARDS (1). This finding, together with results from several other studies comparing ventilation strategies with a low versus a high VT preceding the ARMA trial (2-5) induced a worldwide change in ventilator management. Indeed, ventilation strategies with a low VT became widely used in patients with ARDS, as suggested by the findings of three large service reviews in 2002, 2008 and 2013 (6-8), and the more recent ‘Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE’ (LUNG SAFE) (9).
Two randomized clinical trials, one conducted in the United States (10) and one in The Netherlands (11), showed benefit from VT reduction in patients without or at risk for ARDS. These two studies compared a ventilation strategy with a low VT of 6 mL/kg PBW with a ventilation strategy with a VT of 12 (10) or 10 mL/kg PBW (11), respectively. Ventilation with a low VT resulted in less pulmonary infections (10), and less progression to ARDS (11). It should be mentioned though, that the evidence for benefit from ventilation with a low VT in these two studies was much less convincing then the evidence for benefit in the ARMA trial (1). This was caused, for instance, by the fact that outcome measures were largely subjective in the American study (10) but also because the Dutch ‘High versus Low tidal volumes in patients Not having Acute Lung Injury’ (HiLoNALI) trial was stopped early, as such decreasing its validity (11). Lastly, both studies included relatively small numbers of patients (10,11). Nevertheless, one conventional meta-analysis (12) and two individual patient data (IPD) metanalyses (13,14) confirmed the findings of these two studies.
Certainly driven by the positive results of the ARMA trial in patients with ARDS (1), and maybe also by the findings in the abovementioned investigations in patients without ARDS (10-14), ventilation strategies with a low VT became common also in patients without ARDS. This was demonstrated in the latest service review on ventilation management in resource-rich settings, the ‘Practice of Ventilation in Patients without ARDS’ (PRoVENT) study (15). Probably, service reviews in resource-limited settings will show the same picture (16).
However, meta-analysis in general, and IPD meta-analysis in particular, can suffer from several weaknesses. One of the major caveats of the two IPD meta-analysis mentioned above is that they included patients from investigations performed and published many years apart (13,14). Over this long time span not only ventilator-related strategies, but also strategies unrelated to ventilator management changed (6-8), while outcomes of critically ill patients improved. This improvement may have been related to changes in e.g., VT settings, however, it is not unlikely that factors unrelated to ventilator management played a role herein.
Second, VT varied widely in the two IPD meta-analysis, from very low (e.g., <6 mL/kg PBW) to very high (e.g., >12 mL/kg PBW) (13,14). Consequently, the associations that were found could have been driven more by harm from ventilation strategies using a very high VT, rather than benefit from strategies using a low VT. Here, we should appreciate the fact that ventilation with such high VT fell out of use in patients without ARDS, as clearly illustrated in the publication of the already mentioned PRoVENT study (15).
Third, patients included in the studies pooled in the IPD meta-analysis (13,14) received controlled ventilation more often, and were also switched to supported modes of ventilation much later than patients are nowadays. Of note, this was also true for patients in the two only studies that randomized patients without ARDS to ventilation with a low VT or ventilation with a high VT (10,11). Compared to spontaneous breathing and supported ventilation, controlled ventilation decreases the amount of aerated lung tissue due to inactivity of the diaphragm. This could, at least in theory, increase the harmful effects of ventilation with a high VT.
Thus, it remained uncertain what VT to use in patients without ARDS. Therefore, the PROVE network-investigators designed and performed the ‘PRotective VENTilation in patients without ARDS’ (PReVENT) trial, a randomized study comparing ventilation with a low VT (4 to 6 mL/kg PBW) to ventilation with an intermediate VT (8 to 10 mL/kg PBW) (17). A pragmatic protocol was used with similar targets in both arms for all ventilatory settings and variables, except for the VT and the respiratory rate. Volume-controlled ventilation was used, with the preference to switch early to pressure support ventilation under light sedation. The plateau pressure with volume-controlled ventilation, and the maximum pressure with pressure support ventilation were to be kept below 25 cmH2O. The lowest pressure support level allowed was 5 cmH2O in both ventilation groups. Uncontrollable acidosis, a too high respiratory rate (>35 per minute), and patient-ventilator asynchrony which could not be controlled, e.g., by switching from a controlled to a supported ventilation mode, were the three only reasons for increasing VT in the low VT group. Additional use of sedatives and muscle relaxants, in order to keep VT within the predefined ranges, was never allowed in this study.
The number of ventilator-free days and alive at day 28, the primary endpoint of the PReVENT trial, was not different between the two ventilation groups (29). There were also no differences in mortality, length of stay, and the occurrence of pulmonary complications. Thus, it was concluded that a ventilation strategy targeting a low VT may be as effective as one that targets an intermediate VT with respect to the number of ventilator-free days and alive at day 28, in ICU patients without ARDS who are lightly sedated and quickly weaned to pressure support ventilation.
The study protocol of the PReVENT trial was criticized in an accompanying editorial with the publication of the main results (30). It was commented that the protocol could have been too complex, and not suited to provide a sufficient difference between the two ventilation groups. The first comment is incorrect. The nurses and doctors who used the study protocol found it easy to follow, and adhered to it very well. The second comment seems correct, but only in part. Indeed, one should hold in mind that light sedation was to be practiced if possible, and as mentioned above, muscle relaxants were not allowed to keep VT within the predefined ranges. This means that patients were quickly weaned from controlled ventilation to pressure support ventilation, and indeed much faster than in the two previous studies that compared ventilation with a low versus a high VT in patients without ARDS (10,11). Consequently, VT was less controllable in the PReVENT trial, resulting in less, though still statistically significant contrast between the ventilation groups. Most important however, is that the study protocol with regard to ventilator-related and -unrelated management reflects todays practice.
Another comment on the PReVENT trial was that the study was still underpowered, partly due to the heterogeneous study population included (31). We do not believe this is the case, and of note, in none of the predefined or post-hoc subgroup analyses a difference between was found between the two ventilation groups with regard to the primary endpoint (30,32).
One could argue that the findings of the PReVENT trial mean that a (further) reduction in VT is not needed in patients without ARDS. We warn against this logic. First, the study population of the PReVENT trial included mainly patients who could be quickly weaned to pressure support ventilation. A reduction in VT could still be beneficial in e.g., sicker patients who need controlled ventilation for a longer period of time. Second, ventilation with an intermediate VT in the PReVENT trial resulted in VT of ~9 mL/kg PBW, while the two preceding randomized trials that showed benefit of VT reduction used much higher VT in their control arms [i.e., 12 mL/kg PBW (10) and 10 mL/kg PBW (11), respectively]. Indeed, as the PReVENT trial did not compare ventilation with a low VT to ventilation with a high VT, but ventilation with a low VT to ventilation with an intermediate VT, we should not conclude that ventilation with high VT is safe. Interesting in this context, in absence of studies comparing ventilation with a low VT to ventilation with an intermediate VT in patients with ARDS, no one ever argued that ventilation with an intermediate VT could be equally safe as ventilation with a low VT in patients with ARDS.
In the PReVENT trial, it was also found that patients ventilated with a low VT had a lower driving pressure (ΔP) compared to patients ventilated with an intermediate VT. ΔP, defined as the difference between plateau pressure and the level of positive end-expiratory pressure (PEEP), reflects the strain applied on lung tissue in patients receiving ventilation (18). The issue of ΔP has attained special interest in recent years. In patients with ARDS, ΔP is consistently and repeatedly referred to as the most important ventilator variable associated with relevant outcomes (9,18), even if these patients are put on extracorporeal membrane oxygenation (33). Indeed, a cut-off of 15 cmH2O of ΔP is widely proposed and used at the bedside as a safety limit in patients under invasive ventilation. However, it is important to emphasize that the evidence so far came from observational studies and IPD meta-analysis only. One could argue that the ability of ΔP to predict outcome is attributable to the fact that the variables that define this parameter are themselves highly predictive of survival. Although the concept of aiming for a low ΔP is appealing, it remains uncertain whether it is feasible, and last but not least beneficial to lower ΔP per se rather than VT. Nevertheless, in the PReVENT trial all patients were ventilated with ΔP well below the cut-off mentioned above—even in the patients that were randomized to the ventilation strategy with an intermediate VT.
Ventilation with a low VT could induce alveolar instability, resulting in an increased risk of atelectasis and consequent cyclic opening and closing of alveolar unit. Also, a decrease in functional residual capacity could happen, leading to a rise in ΔP and thus an increased risk of lung injury. PEEP may recruit and stabilize alveoli, thereby decreasing ΔP when a low VT is used (2). PEEP, however, can also lead to overdistension of nondependent lung tissue, which than increases ΔP. Three pivotal clinical trials, the ‘Assessment of Low tidal Volume and Elevated end-expiratory pressure to Obviate Lung Injury’ (ALVEOLI) trial (19), the ‘Lung Open Ventilation to decrease mortality in the acute respiratory distress Syndrome’ (LOVES) trial (20) and the ‘EXpiratory PRESSure’ (EXPRESS) trial (21) failed to show benefit of a ventilation strategy with high PEEP compared to low PEEP in patients with ARDS, while an IPD metaanalysis of these three studies suggested benefit from high PEEP in patients with moderate to severe ARDS (22). However, opposite to expectations, a ventilation strategy with high PEEP, titrated to the compliance of the respiratory system, was shown to be harmful in patients with severe ARDS—indeed, while in the recent ‘Alveolar Recruitment for acute respiratory distress syndrome Trial’ (ART) ventilation with high PEEP resulted in lower ΔP, this strategy increased mortality and morbidity (23). In a way to better titrate high PEEP in patients with ARDS, the more recent ‘Esophageal Pressure-directed VENTilation’ (EPVENT-2) trial compared a strategy with high PEEP guided by frequent esophagus pressure measurements to a strategy in which PEEP was guided by a frequently used PEEP/FiO2 table (34). This study, unfortunately, showed no benefit from a strategy with high PEEP guided by esophagus pressures.
This brings up the question whether or not patients without ARDS benefit from prophylactic high PEEP, or from PEEP at all. Although the results of one Spanish trial suggest that prophylactic high PEEP in patients without ARDS reduces the number of hypoxemia episodes and the incidence of ventilator-associated pneumonia (24), a recent conventional meta-analysis of studies on PEEP clearly demonstrated the lack of evidence for benefit of ventilation with prophylactic high PEEP, and also of ventilation with PEEP at any level, in patients without ARDS (25). Nevertheless, PEEP is increasingly used in these patients, and at increasing levels. The recently started ‘REstricted versus Liberal positive end-expiratory pressure in patients without ARDS’ (RELAx) trial tests the hypothesis that the lowest possible PEEP is sufficient, compared to a standard PEEP of 8 cmH2O in patients without ARDS (26), a level that is currently frequently used in these patients (35).
More recently it has been hypothesized that outcome of ICU patients under invasive ventilation can be explained by the amount of energy that the mechanical ventilation delivers at each breath (27,36). Energy depends on VT, plateau pressure, respiratory rate and air flow, and when the energy is expressed per unit of time it receives the name of ‘mechanical power’. ‘Mechanical power’ thus unifies all ventilator-variables associated with ventilator-induced lung injury. Data from observational studies suggest that ‘mechanical power’ is strongly associated with outcome of patients with ARDS (28), and even of patients without ARDS (27). In the PReVENT trial, probably ‘mechanical power’ was low in most, if not all patients, as VT, and consequent also ΔP, were low. Research on this topic is still in its infancy—we foresee there will be many studies on ‘mechanical power’, in patients with ARDS, and patients without ARDS in the near future.
In conclusion, in patients without ARDS who are lightly sedated and quickly weaned to pressure support ventilation, ventilation with an intermediate VT seems as safe as ventilation with a low VT. Despite these findings, we favor the continued use of ventilation with a low VT, since the PReVENT trial showed no disadvantages from ventilation with a low VT, and patients who need deeper sedation and longer controlled ventilation could still benefit from VT reductions. Finally, a diagnosis of ARDS is frequently missed (9). There is no doubt these latter patients benefit from ventilation with a low VT, leading to ventilation with a lower ΔP and less ‘mechanical power’. Whether or not PEEP should be used prophylactically, and whether low ΔP and low ‘mechanical power’ should be targets of ventilation strategies in patients without ARDS remains highly uncertain.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [Crossref] [PubMed]
- Brochard L, Roudot-Thoraval F, Roupie E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. Am J Respir Crit Care Med 1998;158:1831-8. [Crossref] [PubMed]
- Stewart TE, Meade MO, Cook DJ, et al. Evaluation of a Ventilation Strategy to Prevent Barotrauma in Patients at High Risk for Acute Respiratory Distress Syndrome. N Engl J Med 1998;338:355-61. [Crossref] [PubMed]
- Brower RG, Shanholtz CB, Fessler HE, et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 1999;27:1492-8. [Crossref] [PubMed]
- Esteban A, Anzueto A, Frutos F, et al. Characteristics and Outcomes in Adult Patients Receiving Mechanical Ventilation. JAMA 2002;287:345-55. [Crossref] [PubMed]
- Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med 2008;177:170-7. [Crossref] [PubMed]
- Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188:220-30. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Lee PC, Helsmoortel CM, Cohn SM, et al. Are low tidal volumes safe? Chest 1990;97:430-4. [Crossref] [PubMed]
- Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care 2010;14:R1. [Crossref] [PubMed]
- Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308:1651-9. [Crossref] [PubMed]
- Serpa Neto A, Simonis FD, Barbas CS, et al. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive Care Med 2014;40:950-7. [Crossref] [PubMed]
- Serpa Neto A, Simonis FD, Barbas CS, et al. Lung-Protective Ventilation With Low Tidal Volumes and the Occurrence of Pulmonary Complications in Patients Without Acute Respiratory Distress Syndrome: A Systematic Review and Individual Patient Data Analysis. Crit Care Med 2015;43:2155-63. [Crossref] [PubMed]
- Serpa Neto A, Barbas CSV, Simonis FD, et al. Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study. Lancet Respir Med 2016;4:882-93. [Crossref] [PubMed]
- Pisani L, Algera AG, Serpa Neto A, et al. PRactice of VENTilation in Middle-Income Countries (PRoVENT-iMIC): rationale and protocol for a prospective international multicentre observational study in intensive care units in Asia. BMJ Open 2018;8:e020841. [Crossref] [PubMed]
- Simonis FD, Binnekade JM, Braber A, et al. PReVENT - protective ventilation in patients without ARDS at start of ventilation: Study protocol for a randomized controlled trial. Trials 2015;16:226. [Crossref] [PubMed]
- Amato MBP, Meade MO, Slutsky AS, et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus Lower Positive End-Expiratory Pressures in Patients with the Acute Respiratory Distress Syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. [Crossref] [PubMed]
- Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. [Crossref] [PubMed]
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. [Crossref] [PubMed]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- Manzano F, Fernández-Mondéjar E, Colmenero M, et al. Positive-end expiratory pressure reduces incidence of ventilator-associated pneumonia in nonhypoxemic patients. Crit Care Med 2008;36:2225-31. [Crossref] [PubMed]
- Serpa Neto A, Filho RR, Cherpanath T, et al. Associations between positive end-expiratory pressure and outcome of patients without ARDS at onset of ventilation: a systematic review and meta-analysis of randomized controlled trials. Ann Intensive Care 2016;6:109. [Crossref] [PubMed]
- Algera AG, Pisani L, Bergmans DC, et al. RELAx - REstricted versus Liberal positive end-expiratory pressure in patients without ARDS: protocol for a randomized controlled trial. Trials 2018;19:272. [Crossref] [PubMed]
- Serpa Neto A, Deliberato RO, Johnson AEW, et al. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med 2018;44:1914-22. [Crossref] [PubMed]
- Zhang Z, Zheng B, Liu N, et al. Mechanical power normalized to predicted body weight as a predictor of mortality in patients with acute respiratory distress syndrome. Intensive Care Med 2019;45:856-64. [Crossref] [PubMed]
- Writing Group for the PReVENT Investigators, Simonis FD, Serpa Neto A, et al. Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS: A Randomized Clinical Trial. JAMA 2018;320:1872-80. [Crossref] [PubMed]
- Rubenfeld GD, Shankar-Hari M. Lessons From ARDS for Non-ARDS Research Remembrance of Trials Past. JAMA 2018;320:1863-5. [Crossref] [PubMed]
- Slagt C, van Eijk L. Tidal Volume Ventilation Strategy in ICU Patients Without ARDS. JAMA 2019;321:1311-2. [Crossref] [PubMed]
- Neto AS, Simonis FD, Schultz MJ. Tidal Volume Ventilation Strategy in ICU Patients Without ARDS-Reply. JAMA 2019;321:1312-3. [Crossref] [PubMed]
- Serpa Neto A, Schmidt M, Azevedo LC, et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis. Intensive Care Med 2016;42:1672-84. [Crossref] [PubMed]
- Beitler JR, Sarge T, Banner-Goodspeed VM, et al. Effect of Titrating Positive End-Expiratory Pressure (PEEP) With an Esophageal Pressure-Guided Strategy vs an Empirical High PEEP-Fio2 Strategy on Death and Days Free From Mechanical Ventilation Among Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2019;321:846-57. [Crossref] [PubMed]
- van IJzendoorn MC, Koopmans M, Strauch U, et al. Ventilator setting in ICUs: comparing a Dutch with a European cohort. Neth J Med 2014;72:473-80. [PubMed]
- Gattinoni L, Tonetti T, Cressoni M, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 2016;42:1567-75. [Crossref] [PubMed]


