Pneumothorax in cystic fibrosis
Introduction
Cystic fibrosis (CF) is an autosomal genetic disease affecting a variety of organs, but especially involving respiratory tract and pancreas. The lungs undergo gradual destruction, as an effect of persistent microbial colonization, chronic inflammation and recurrent infections caused by highly pathogenic bacteria, such as Pseudomonas aeruginosa and Staphylococcus aureus. Several constituents of the innate immunity are also affected by the disease (1). As a consequence, despite the amazing progress in treating CF-related lung disease, it still accounts for nearly 85% of the mortality (2).
The spectrum of the clinical manifestations of the disease is explained by the presence of mutations of the gene for cystic fibrosis transmembrane conductance regulator (CFTR), inherited from each parent (3). CFTR regulates a c-AMP anion channel on the apical surfaces of epithelial cells, and reduced or abolished activity of this protein results to defected transport of chloride, HCO3 and sodium ions across membranes, and the accumulation of thick mucus (4). The airways of the affected lungs are clogged by purulent secretions and deformed due to the development of bronchiectatic lesions, while in the parenchyma the formation of multiple cavitations or cysts, areas of bronchiolar consolidation, fibrosis and air-trapping compromise respiratory adequacy (5).
Epidemiology
Pneumothorax is a known serious complication in CF patients (6). In a retrospective observational cohort study, Flume et al. reviewed the data of 28,858 patients with CF who had been followed up over 10 years at CF centers across the United States. Pneumothorax occurred with an average annual incidence of 0.64% and 3.4% of the patients overall. The same study was able to recognize a number of risk factors associated with an increased occurrence of pneumothorax, including the presence of P. aeruginosa. Burkholderia cepacia or Aspergillus in sputum cultures, FEV1 <30% of predicted, enteral feeding, pancreatic insufficiency, allergic bronchopulmonary aspergillosis (ABPA) and massive hemoptysis. There was no increased occurrence by sex, but pneumothorax was more prevalent in older patients (median age 21 years) with more sever pulmonary impairment and was not only a cause of increased morbidity, but was responsible for increased 2-year mortality (7). Actually much older studies have concluded that the incidence of pneumothorax in CF patients has increased as improved treatment has resulted in prolonged survival (8-10). A review covering 26 years of experience from a single center reported 99 patients with at least one episode of pneumothorax out of 1,268 patients with CF who were followed between 1959 and 1987 (11). Another, though smaller retrospective study reported 17 episodes of pneumothorax in 11 patients from a cohort of approximately 500 children with CF living in the area of Victoria, Australia, over a 15-year period. The authors found only a slight higher than the predicted decline in lung function (6% vs. 4%) over the 2-year period around pneumothorax. Nevertheless, they suggested that increased rate was more likely attributed to P. aeruginosa and B. cepacia colonization than to pneumothorax per se (12).
It is evident that the estimation of the incidence of pneumothorax in CF patients varies, depending not only on the age of the patients (e.g., children vs. adults) and the severity of the lung impairment in the included cohort of patients, but also on the efficacy of the overall management of the respiratory component of the disease, a fact that is reflected on the year of publication (old vs. recent).
Etiology
Both structural impairment and altered airflow dynamics in the lungs could considered as predisposing factors for the occurrence of spontaneous pneumothorax in CF patients. Rapture of subpleural blebs and bullae in the visceral pleura apparently represent a common underlying mechanism. Nevertheless, there is a poor correlation between the presence of blebs or cysts and pneumothorax in CF patients (13). In addition, endobronchial obstruction and inflammation due to accumulation of viscous secretions and inflammatory cells, especially macrophages, cause air-trapping and induce overpressure in the alveolar tissue, resulting in rupture of pulmonary parenchyma (14). It is reported that shear forces generated during abrupt acceleration/deceleration of a CF patient, increased mechanical stress on the pleura, causing bilateral pneumothorax (15).
Although a substantial percentage (8-21%) of CF patients are smokers and smoking is a well-known factor related to spontaneous pneumothorax, there is a lapse of reported cases of such etiology (16-19). It has been shown that inhaled medications (e.g., dornase-α, tobramycin) could increase the risk of pneumothorax, probably due to the acute decrease in FEV1 following inhalation (20). Also, transplant listed CF patients requiring non-invasive positive pressure ventilation (NIPPV), are in increased risk for pneumothorax. In such challenging clinical situation, pneumothorax may cause significant deterioration of the respiratory status necessitating the withdrawal of NIPPV (21).
ABPA is prevalent (7.8%) in cystic fibrosis and is clearly related to respiratory complications, including pneumothorax (22).
Thoracic endometriosis is a rare condition characterized by catamenial hemoptysis and pneumothorax. This condition is observed, albeit extremely rarely, in CF patients, for which could present a life threatening event (23).
Clinical presentation
In one pioneering publication including 49 patients who have attended the Brompton Hospital between 1964 and 1969 it was spotted that spontaneous pneumothorax could be either a terminal event or an incidental finding which required no specific treatment (24). The majority of the patients experience pain, and shortness of breath, while some of them die acutely (7). Development of acute respiratory failure requiring ventilatory support is possible. Sood et al. reported that 3 (2%) out of 136 admissions of CF patients in the ICU during a 9-year period were due to pneumothorax. One patient required intubation and all three improved and were discharged after an average ICU stay of 1.6 days (25). However, a more recent retrospective analysis from a single center provided data showing that only 59% of 22 patients intubated for pneumothorax and/or hemoptysis survived to hospital discharge (26).
Diagnosis
When clinically suspected, pneumothorax should diagnosed by the proper imaging technique. Performing a chest radiograph is the simplest method to diagnose pneumothorax (27). Diagnosis may be difficult in CF patients when relying on plain radiography alone, due to the presence of cysts and bullae. CT scanning of the chest facilitates not only diagnosis but also the selection of appropriate management (28,29) (Figure 1).
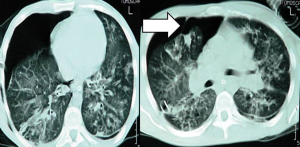
The use of MRI in the clinical management of complications of advanced CF, such as pneumothorax, is of limited value (30).
Management
The aim of treatment in CF patients is the safe and effective resolution of pneumothorax with prevention of recurrence (31). Historically, a variety of therapeutic interventions were employed, including sclerosant agents (quinacrine, silver nitrate, iodine, talc, etc.), pleural abrasion, intercostal drainage, pleurectomy and Heimlich flutter valve (32-37). Most of these methods were quite successful, but there were significant differences in the recurrence rates. Quinacrine sclerosis and, particularly, parietal pleurectomy appeared to be among the most effective methods of management (38,39). Pleural suction drainage was reported to have the lowest long-term success rate (33).
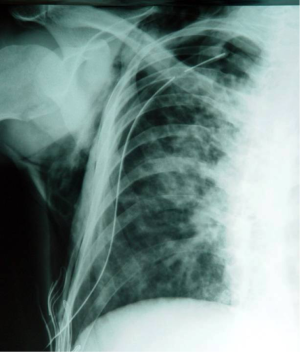
However, according to the British Thoracic Society (BTS) guidelines for the management of spontaneous pneumothorax [2003], the treatment of pneumothorax for patients with CF is similar to that for non-CF patients, giving emphasis to early and aggressive treatment and suggesting that surgical intervention should be considered after the first episode, provided that the patient is fit for the procedure (40). BTS guidelines state that a small pneumothorax without symptoms can be observed or aspirated, while large pneumothoraces require treatment with intercostal tube drainage. It is stressed that the air-leak is usually from the upper lobes making important the siting of the tube in the correct place (Figure 2). The authors consider that patients with recurrent pneumothoraces should undergo partial pleurectomy, which has a success rate of 95%. On the contrary, patients that are too ill for surgical intervention, intubation and suction present the best option, having in mind that it can take 2-3 weeks for the lung to re-expand. The 2003 guidelines accepted the conclusions of Noyes and Orenstein [1992] that administration of sclerosant agents present, at least partial, contraindication for subsequent lung transplantation, as they make lung removal more difficult, prolong the ischemic time for the donor lungs and can cause excessive bleeding (41). Notably, others suggested the avoidance of therapeutic pleurectomy due to the same reasons (42). Although BTS guidelines were updated in 2010 (43) without major differences from the previous edition, they highlighted that pleural procedures (including pleurodesis), do not have a significant adverse effect on the outcome of subsequent lung transplantation, adopting thus, the relevant conclusions of a more recent comparative study (44). Despite that optimism, a more conservative approach suggests that each patient should be considered individually when evaluating the impact of the previous pleural procedure on the candidacy for lung transplantation (45). Especially pleurectomy is considered by thoracic surgeons as a major obstacle to future operative procedures, including transplantation (46).
These treatment suggestions were somewhat challenged by certain experts arguing the superiority of video-assisted thoracoscopic surgery (VATS) (47). Their major arguments are focused on better safety, shorter postoperative period and less pain medication required, despite the marginally higher recurrence rates observed with VATS. These arguments are also supported by previous studies including small series of CF patients, reporting excellent results with VATS, without complications or subsequent recurrences of the pneumothorax (48,49).
Concurrently to BTS 2010 guidelines, an ad hoc American committee published clinical practice guidelines for cystic fibrosis pulmonary therapies. According to their statement, all CF patients with a large pneumothorax should be admitted to the hospital and have a chest tube placed. In case of recurrent large pneumothorax the patient should undergo, preferably surgical, pleurodesis. As for the pneumothorax cases being under ventilatory support with BiPAP, the panel felt that discontinuation of non-invasive ventilation is the preferred option. Finally, the panel suggested that CF patients with pneumothorax should neither fly on a plane, nor perform spirometry or lift weights for 2 weeks after the pneumothorax has resolved (50).
Autologus “blood patching” pleurodesis has been used successfully for the treatment of persistent air-leak in patients with spontaneous pneumothorax but there is very limited experience with cystic fibrosis patients. A case report of a young CF patient underlines the possibility for development of tension pneumothorax during the procedure, secondary to blood clot (51). Also, biological glue has been tried successfully (52).
The debate of chemical versus surgical pleurodesis (53) was reviewed by the Cochrane Collaboration which confirmed the lack of randomized controlled studies in this population (54).
Lung transplantation is considered as the ultimate solution to the problem of refractory and/or recurrent pneumothorax (55).
Continuation or withholding of physiotherapy during or immediately after the management of pneumothorax is an issue. According to the above mentioned American guidelines, some airway clearance techniques such as positive expiratory pressure and intrapulmonary percussive ventilation should not be used. Alternative airway clearance therapies include high-frequency chest compression, active cycle breathing and autogenic drainage (56). Nevertheless, once the therapeutic intervention has been undertaken, physiotherapy should focus on maintaining effective sputum clearance to prevent atelectasis and lobar collapse due to obstructive secretions. In addition, inspired oxygen should be adequately humidified and effective analgesia should be administered to relieve aggravation of pain caused by the chest tube during airway clearance techniques, leading to sputum retention (57,58).
Prognosis
Pneumothorax is a cause of significant short- and long-term morbidity and mortality in CF patients. It is reported that 50-90% of the patients will suffer a recurrence (defined as a pneumothorax that develops on the ipsilateral side more than 7 days after the resolution of the first event) and 46% will experience a subsequent contralateral pneumothorax (7,35). According to Flume et al. attributable mortality is estimated at 6.3-14.3%, while the 2-year mortality rate in patients following a pneumothorax is 48.6% (59). An analysis of data from the European Epidemiologic Registry of Cystic Fibrosis (ERCF) including more than 7,000 patients, was able to demonstrate that pneumothorax was among the factors associated with poor pulmonary function, as reflected by FEV1 >10% of predicted values (60). It should be noted that as it was previously showed, FEV1 is the strongest predictor of survival and one of the major indicators for lung transplantation (61,62). Moreover, ERCF data covering the decade 1990-1999 estimate the mortality of CF patients experiencing pneumothorax at 48.6%, compared to 12.2% of patients without pneumothorax (54). As reported by Spector and Stern (11), the median survival after the first pneumothorax is 29.9 months and a more recent study confirmed that patients who had suffered a pneumothorax prior to referral to the adult clinic were less likely to become long-term survivors (i.e., aged >40 years) (63). An Australian study focused on CF patients requiring surgical intervention, reported 50% mortality in children with pneumothorax (64). Finally, according to a study in German CF patients, recent pneumothorax precipitates symptoms of anxiety (65-79), deteriorating their quality of life (80-93).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med 2012;18:509-19. [PubMed]
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: 2005 Annual data report to the center directors. Bethesda, MD: Cystic Fibrosis Foundation; 2006
- Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066-73. [PubMed]
- Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev 1999;79:S3-22. [PubMed]
- Aziz ZA, Davies JC, Alton EW, et al. Computed tomography and cystic fibrosis: promises and problems. Thorax 2007;62:181-6. [PubMed]
- Bernard E, Israel L, Debris MM, et al. Mucoviscidosis and idiopathic spontaneous pneumothorax. J Fr Med Chir Thorac 1962;16:105-9. [PubMed]
- Flume PA, Strange C, Ye X, et al. Pneumothorax in cystic fibrosis. Chest 2005;128:720-8. [PubMed]
- Tribble CG, Selden RF, Rodgers BM. Talc poudrage in the treatment of spontaneous pneumothoraces in patients with cystic fibrosis. Ann Surg 1986;204:677-80. [PubMed]
- Boat TF. Pneumothorax in cystic fibrosis. JAMA 1969;209:1498-504. [PubMed]
- Tomashefski JF Jr, Bruce M, Stern RC, et al. Pulmonary air cysts in cystic fibrosis: relation of pathologic features to radiologic findings and history of pneumothorax. Hum Pathol 1985;16:253-61. [PubMed]
- Spector ML, Stern RC. Pneumothorax in cystic fibrosis: a 26-year experience. Ann Thorac Surg 1989;47:204-7. [PubMed]
- Hafen GM, Ukoumunne OC, Robinson PJ. Pneumothorax in cystic fibrosis: a retrospective case series. Arch Dis Child 2006;91:924-5. [PubMed]
- McLaughlin FJ, Matthews WJ Jr, Strieder DJ, et al. Pneumothorax in cystic fibrosis: management and outcome. J Pediatr 1982;100:863-9. [PubMed]
- Schramel FM, Postmus PE, Vanderschueren RG. Current aspects of spontaneous pneumothorax. Eur Respir J 1997;10:1372-9. [PubMed]
- Murphy D, O’Mahony M, Logan P, et al. Bilateral pneumothoraces following a bungee jump in a patient with cystic fibrosis. Respiration 2006;73:113. [PubMed]
- Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest 1987;92:1009-12. [PubMed]
- Jansveld CA, Dijkman JH. Primary spontaneous pneumothorax and smoking. Br Med J 1975;4:559-60. [PubMed]
- Britto MT, Garrett JM, Dugliss MA, et al. Risky behavior in teens with cystic fibrosis or sickle cell disease: a multicenter study. Pediatrics 1998;101:250-6. [PubMed]
- Verma A, Clough D, McKenna D, et al. Smoking and cystic fibrosis. J R Soc Med 2001;94 Suppl 40:29-34. [PubMed]
- Alothman GA, Alsaadi MM, Ho BL, et al. Evaluation of bronchial constriction in children with cystic fibrosis after inhaling two different preparations of tobramycin. Chest 2002;122:930-4. [PubMed]
- Haworth CS, Dodd ME, Atkins M, et al. Pneumothorax in adults with cystic fibrosis dependent on nasal intermittent positive pressure ventilation (NIPPV): a management dilemma. Thorax 2000;55:620-2. [PubMed]
- Mastella G, Rainisio M, Harms HK, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis. A European epidemiological study. Epidemiologic Registry of Cystic Fibrosis. Eur Respir J 2000;16:464-71. [PubMed]
- Parker CM, Nolan R, Lougheed MD. Catamenial hemoptysis and pneumothorax in a patient with cystic fibrosis. Can Respir J 2007;14:295-7. [PubMed]
- Mitchell-Heggs PF. Spontaneous pneumothorax in cystic fibrosis. Thorax 1970;25:256. [PubMed]
- Sood N, Paradowski LJ, Yankaskas JR. Outcomes of intensive care unit care in adults with cystic fibrosis. Am J Respir Crit Care Med 2001;163:335-8. [PubMed]
- Jones A, Bilton D, Evans TW, et al. Predictors of outcome in patients with cystic fibrosis requiring endotracheal intubation. Respirology 2013;18:630-6. [PubMed]
- Phillips GD, Trotman-Dickenson B, Hodson ME, et al. Role of CT in the management of pneumothorax in patients with complex cystic lung disease. Chest 1997;112:275-8. [PubMed]
- Greene KE, Takasugi JE, Godwin JD, et al. Radiographic changes in acute exacerbations of cystic fibrosis in adults: a pilot study. AJR Am J Roentgenol 1994;163:557-62. [PubMed]
- Matrunola M, Polettini E, Roggini M, et al. High resolution computerized tomography in cystic fibrosis. Clinico-radiologic correlations in 25 patients. Clin Ter 1995;146:133-40. [PubMed]
- Grum CM, Lynch JP 3rd. Chest radiographic findings in cystic fibrosis. Semin Respir Infect 1992;7:193-209. [PubMed]
- Mohan K, Ledson MJ, Walshaw MJ, et al. Simultaneous bilateral spontaneous pneumothorax in an adult patient with cystic fibrosis. J Bras Pneumol 2009;35:194-6. [PubMed]
- Penketh AR, Knight RK, Hodson ME, et al. Management of pneumothorax in adults with cystic fibrosis. Thorax 1982;37:850-3. [PubMed]
- Aebersold A, Schaad UB. Pneumothorax in cystic fibrosis. Schweiz Med Wochenschr 1991;121:174-81. [PubMed]
- Kattwinkel J, Taussig LM, McIntosh CL, et al. Intrapleural instillation of quinacrine for recurrent pneumothorax. Use in a patient with cystic fibrosis. JAMA 1973;226:557-9. [PubMed]
- Rich RH, Warwick WJ, Leonard AS. Open thoracotomy and pleural abrasion in the treatment of spontaneous pneumothorax in cystic fibrosis. J Pediatr Surg 1978;13:237-42. [PubMed]
- Tribble CG, Selden RF, Rodgers BM. Talc poudrage in the treatment of spontaneous pneumothoraces in patients with cystic fibrosis. Ann Surg 1986;204:677-80. [PubMed]
- Edenborough FP, Hussain I, Stableforth DE. Use of a Heimlich flutter valve for pneumothorax in cystic fibrosis. Thorax 1994;49:1178-9. [PubMed]
- McLaughlin FJ, Matthews WJ Jr, Strieder DJ, et al. Pneumothorax in cystic fibrosis: management and outcome. J Pediatr 1982;100:863-9. [PubMed]
- Mitchell-Heggs PF, Batten JC. Pleurectomy for spontaneous pneumothorax in cystic fibrosis. Thorax 1970;25:165-71. [PubMed]
- Henry M, Arnold T, Harvey J, et al. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52. [PubMed]
- Noyes BE, Orenstein DM. Treatment of pneumothorax in cystic fibrosis in the era of lung transplantation. Chest 1992;101:1187-8. [PubMed]
- Seddon DJ, Hodson ME. Surgical management of pneumothorax in cystic fibrosis. Thorax 1988;43:739-40. [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [PubMed]
- Curtis HJ, Bourke SJ, Dark JH, et al. Lung transplantation outcome in cystic fibrosis patients with previous pneumothorax. J Heart Lung Transplant 2005;24:865-9. [PubMed]
- Rosenblatt RL. Lung transplantation in cystic fibrosis. Respir Care 2009;54:777-86; discussion 786-7. [PubMed]
- Rolla M, D’Andrilli A, Rendina EA, et al. Cystic fibrosis and the thoracic surgeon. Eur J Cardiothorac Surg 2011;39:716-25. [PubMed]
- Ng CS, Wan S, Yim AP. Paradigm shift in surgical approaches to spontaneous pneumothorax: VATS. Thorax 2004;59:357-author reply 357. [PubMed]
- Cannon WB, Vierra MA, Cannon A. Thoracoscopy for spontaneous pneumothorax. Ann Thorac Surg 1993;56:686-7. [PubMed]
- Stringel G, Amin NS, Dozor AJ. Video-assisted thoracoscopy in the management of recurrent spontaneous pneumothorax in the pediatric population. JSLS 1999;3:113-6. [PubMed]
- Flume PA, Mogayzel PJ Jr, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med 2010;182:298-306. [PubMed]
- Williams P, Laing R. Tension pneumothorax complicating autologous “blood patch” pleurodesis. Thorax 2005;60:1066-7. [PubMed]
- Garske LA, Tam RK, Windsor MF, et al. Novel application of biological glue in the management of a complicated pneumothorax in cystic fibrosis. Pediatr Pulmonol 2002;34:138-40. [PubMed]
- Rodgers BM, Tribble CG. Pneumothorax in cystic fibrosis. Ann Thorac Surg 1989;48:743-4. [PubMed]
- Amin R, Noone PG, Ratjen F. Chemical pleurodesis versus surgical intervention for persistent and recurrent pneumothoraces in cystic fibrosis. Cochrane Database Syst Rev 2012;12:CD007481. [PubMed]
- Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. [PubMed]
- Flume PA. Pulmonary complications of cystic fibrosis. Respir Care 2009;54:618-27. [PubMed]
- Prasad SA, Tannenbaum EL, Mikelsons C. Physiotherapy in cystic fibrosis. J R Soc Med 2000;93 Suppl 38:27-36. [PubMed]
- Newton TJ. Respiratory care of the hospitalized patient with cystic fibrosis. Respir Care 2009;54:769-75; discussion 775-6. [PubMed]
- Flume PA. Pneumothorax in cystic fibrosis. Chest 2003;123:217-21. [PubMed]
- Navarro J, Rainisio M, Harms HK, et al. Factors associated with poor pulmonary function: cross-sectional analysis of data from the ERCF. European Epidemiologic Registry of Cystic Fibrosis. Eur Respir J 2001;18:298-305. [PubMed]
- Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970-1989. Am J Epidemiol 1996;143:1007-17. [PubMed]
- Kerem E, Reisman J, Corey M, et al. Prediction of mortality in patients with cystic fibrosis. N Engl J Med 1992;326:1187-91. [PubMed]
- Simmonds NJ, Macneill SJ, Cullinan P, et al. Cystic fibrosis and survival to 40 years: a case-control study. Eur Respir J 2010;36:1277-83. [PubMed]
- Escobar MA, Grosfeld JL, Burdick JJ, et al. Surgical considerations in cystic fibrosis: a 32-year evaluation of outcomes. Surgery 2005;138:560-71; discussion 571-2. [PubMed]
- Goldbeck L, Besier T, Hinz A, et al. Prevalence of symptoms of anxiety and depression in German patients with cystic fibrosis. Chest 2010;138:929-36. [PubMed]
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6 Suppl 1:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6 Suppl 1:S7-20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6 Suppl 1:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6 Suppl 1:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6 Suppl 1:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6 Suppl 1:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6 Suppl 1:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6 Suppl 1:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6 Suppl 1:S162-6. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6 Suppl 1:S152-61. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6 Suppl 1:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4 Suppl 1:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]
- Papaioannou M, Pitsiou G, Manika K, et al. COPD Assessment Test: A Simple Tool to Evaluate Disease Severity and Response to Treatment. COPD 2014;11:489-95. [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-107. [PubMed]
- Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014;6 Suppl 1:S146-51. [PubMed]
- Zarogoulidis P, Porpodis K, Kioumis I, et al. Experimentation with inhaled bronchodilators and corticosteroids. Int J Pharm 2014;461:411-8. [PubMed]
- Bai C, Huang H, Yao X, et al. Application of flexible bronchoscopy in inhalation lung injury. Diagn Pathol 2013;8:174. [PubMed]
- Zarogoulidis P, Kioumis I, Porpodis K, et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 2013;7:1115-34. [PubMed]
- Zarogoulidis P, Pataka A, Terzi E, et al. Intensive care unit and lung cancer: when should we intubate? J Thorac Dis 2013;5 Suppl 4:S407-12. [PubMed]
- Hohenforst-Schmidt W, Petermann A, Visouli A, et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug Des Devel Ther 2013;7:627-33. [PubMed]
- Zarogoulidis P, Kontakiotis T, Tsakiridis K, et al. Difficult airway and difficult intubation in postintubation tracheal stenosis: a case report and literature review. Ther Clin Risk Manag 2012;8:279-86. [PubMed]
- Zarogoulidis P, Tsakiridis K, Kioumis I, et al. Cardiothoracic diseases: basic treatment. J Thorac Dis 2014;6 Suppl 1:S1. [PubMed]
- Kolettas A, Grosomanidis V, Kolettas V, et al. Influence of apnoeic oxygenation in respiratory and circulatory system under general anaesthesia. J Thorac Dis 2014;6 Suppl 1:S116-45. [PubMed]
- Turner JF, Quan W, Zarogoulidis P, et al. A case of pulmonary infiltrates in a patient with colon carcinoma. Case Rep Oncol 2014;7:39-42. [PubMed]
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol 2013;8:194. [PubMed]
- Tsakiridis K, Zarogoulidis P. An interview between a pulmonologist and a thoracic surgeon-Pleuroscopy: the reappearance of an old definition. J Thorac Dis 2013;5 Suppl 4:S449-51. [PubMed]
- Huang H, Li C, Zarogoulidis P, et al. Endometriosis of the lung: report of a case and literature review. Eur J Med Res 2013;18:13. [PubMed]


