Multidisciplinary treatment of thymic neuroendocrine tumors: surgery remains a key component
Introduction
Thymic neuroendocrine tumors (NETs) represent a rare subset of thymic neoplasms, accounting for approximately 2–5% of all thymic tumors (1). Additionally, while NETs are most frequently identified in the terminal ileum and appendix, NET of the thymus account for 0.4% of these tumors (2). These tumors are reported to occur more frequently in men, with a three-to-one incidence relative to women, and the mean age at presentation is 54 years (3,4). Thymic NET may be associated with multiple endocrine neoplasia type 1 (MEN1) in about 25% of cases, and furthermore may be either nonfunctional or functional, with functional tumors most commonly manifesting as Cushing Syndrome secondary to ectopic adrenocorticotropic hormone (ACTH) production (5,6).
Prior investigations have demonstrated the aggressive nature of thymic NET, with variable overall survival (OS) rates reported. In a review of 157 patients with thymic NET spanning 20 years, 5-year survival was reported to be 27% (3). Recent evidence has suggested more optimistic survival rates, which may be dependent upon stage, grade, size, surgical resection, and functional status (4,7). Importantly, a report of the experience of 160 patients included in the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a 5-year survival rate of 80% for patients with local disease only at the time of diagnosis, while another investigation reported a 79% 5-year survival (4,6). Similarly, differential recurrence-free survival (RFS) has also been demonstrated based upon tumor differentiation, with 5-year RFS ranging from 50% for low-grade tumors to 0% for high-grade tumors (8). Owing to the variable biology, some authors have suggested that the terminology of “carcinoid” be abandoned in favor of evaluating these tumors as part of a continuum of neuroendocrine carcinomas (7).
Classification of thymic NET has undergone several changes in recent decades. Formerly called “epithelial thymomas,” Rosai and Higa later renamed these tumors as “thymic carcinoids” in 1972 after close examination of 8 cases (9). The World Health Organization (WHO) subsequently categorized these tumors into either well-differentiated (typical and atypical carcinoids) or poorly-differentiated (large cell and small cell neuroendocrine carcinoma), with typical and atypical carcinoids representing low and intermediate-grade tumors, and large and small cell carcinomas categorized as high-grade (10,11). Additional immunohistochemical evaluation has aided in identifying these tumors types, differentiating more benign carcinoids from carcinomas with more aggressive biology and behavior (12).
As understanding of the classifications and variable behaviors of thymic NET has developed over the last several decades, few authors have reported the experience of patients in small case series or heterogeneous population-level databases. However, outcomes of a relatively large sample from a single center have not been previously reported. Therefore, we sought to describe our experience with management of thymic NET, and to determine predictors of recurrence and survival.
Methods
Patient population
A retrospective review was performed at a single quaternary referral center (The University of Texas MD Anderson Cancer Center) after Institutional Review Board approval with a waiver of informed consent (PA15-0742). Patients presenting for evaluation of thymic NET from January 1, 1975 to May 31, 2018 were identified using natural language processing techniques which have been previously described (13).
Demographic and clinicopathologic variables were evaluated to determine the effect on survival and recurrence. Tumor grade was determined according to the WHO 2015 classification for thymic neoplasms, and tumors were categorized as either typical carcinoid (low-grade), atypical carcinoid (intermediate-grade), or high-grade neuroendocrine carcinoma (10) (Table 1). Tumor size was defined by final pathologic diameter. In the absence of surgical resection or unavailable pathology reporting of tumor size, the diameter was determined by baseline axial imaging, such as computed tomography. OS was defined as the time from the time of surgery or completion of either definitive chemotherapy and/or radiation therapy to death from any cause. RFS was defined as the time from surgical resection to any recurrence or death. Patients surviving at the end of the study period without an event were censored at the date of last follow-up.

Full table
Statistical analysis
Several factors were evaluated in separate univariable followed by multivariable Cox proportional hazards regression analyses to identify predictors of two endpoints: OS and RFS. Variables with a P value less than 0.25 on univariable analysis were included in the respective multivariable Cox regression model. A P value of less than 0.05 was considered to be significant on multivariable analysis.
Using factors determined to be significant in multivariable analyses for OS and RFS, time-to-event outcomes were analyzed using the Kaplan-Meier method, and the log-rank test was used to compare survival and recurrence times between groups of patients. Analyses were performed using SPSS version 24 (IBM, Armonk, NY, USA).
Results
Demographic, surgical, and pathological characteristics
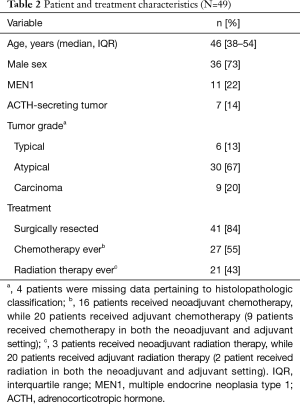
From January 1, 1975 to May 31, 2018, 49 patients were evaluated at The University of Texas MD Anderson Cancer Center for a diagnosis of thymic NET. Patient and treatment characteristics are shown in Table 2. The majority of patients were male with a median age of 46 years [interquartile range (IQR) 38–54]. Eleven (22%) patients were identified to have thymic NET in association with MEN1, and a minority were found to have functional tumors producing ACTH. Twelve patients had Ki67 reported, with a median value of 13% (range, 3–50%).
Full table
With respect to disease management, 41 (84%) patients underwent surgical resection. Overall, chemotherapy and radiation therapy were employed in 27 (55%) and 21 (43%) patients, respectively, as either definitive or adjunctive disease treatment. The mean (± standard deviation) radiation dose administered was 56±8 Gy. Of patients with available chemotherapeutic records, the vast majority received a platinum-based agent (22/27, 81%), typically in conjunction with etoposide (19/27, 70%). In response to definitive or neoadjuvant chemotherapy (n=16), 4 patients were observed to have responsive tumors with deceasing size following restaging examination, while 9 patients had stable disease; tumor response could not be determined for 3 patients. Upon imaging evaluation at the time of diagnosis, the majority of patients (22, 45%) had only local disease while regional disease was observed in 18 (37%) instances. Nine (18%) patients were reported to have metastatic disease at presentation, including 5 with bone metastases and 4 with pulmonary metastases. Using contemporary Masaoka-Koga staging, patients had stage I, II, III, and IV disease in 2 (4%), 7 (14%), 7 (14%), 27 (55%) instances; stage was unable to be determined from the records of 6 (12%) patients. While 9 patients had distant disease at the time of diagnosis, the remainder of those with Masaoka-Koga stage IV were classified as stage IVb due to nodal involvement. Of the 8 patients in whom resection was not pursued, 4 had distant disease at the time of evaluation.
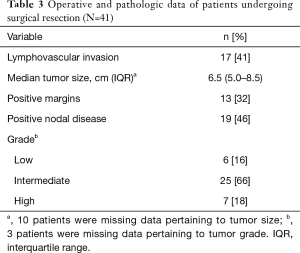
Of the 41 patients who underwent surgical resection, the median tumor size was 6.5 centimeters (IQR, 5.0–8.5) (Table 3). Lymphovascular invasion (LVI) and positive margins were noted in the surgical specimen of 17 (41%) and 13 (32%) patients, respectively. Due to bulky adenopathy upon surgical exploration, 24 (49%) patients underwent nodal dissection with 19 (46%) of these having positive nodal disease. Most tumors were classified as atypical carcinoids (intermediate-grade), while 7 (18%) were deemed to be high-grade. Six (16%) patients were noted to have typical (low-grade) tumors. Regarding treatment strategies, 14 (34%) patients underwent surgical resection alone (Table 4). A majority of patients received adjunctive chemotherapy and/or radiation therapy in addition to surgical resection including 3 (7%) who received neoadjuvant therapy and 18 (44%) who underwent adjuvant therapy. There were 5 (12%) patients who were given either chemotherapy or radiation therapy in both the neoadjuvant and adjuvant settings.
Full table

Full table
Analysis of survival
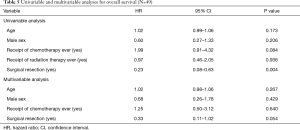
The median survival time and 5-year survival rate were 83.7 months [95% confidence interval (CI), 57.3–110.1 months] and 68%. Univariable and multivariable analyses were conducted to determine predictors of survival (Table 5). Age, sex, chemotherapy use, and surgical resection were included in multivariable analysis, whereupon no demographic or clinicopathologic factors were found to be associated with OS. However, improved OS in patients undergoing surgical resection of the primary tumor closely approached statistical significance (P=0.054).
Full table
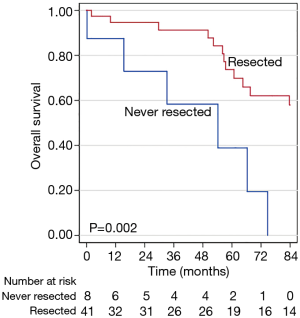
In unadjusted Kaplan-Meier survival analysis, surgical resection was associated with a longer OS, with 5-year survival rates of 73% vs. 41% for those who did and did not undergo surgical resection (P=0.002) (Figure 1). Furthermore, while receipt of any chemotherapy (P=0.324) or radiation therapy (P=0.594) did not appear to be associated with survival benefit in Kaplan-Meier analyses, patients who received any neoadjuvant therapy demonstrated poorer OS than those who did not (P=0.011). Use of any adjuvant therapy did not appear to be associated with differences in OS times (P=0.861). Additionally, those with ACTH-producing tumors did suffer earlier death when compared to patients without hormone-producing malignancies.
Analysis of recurrence
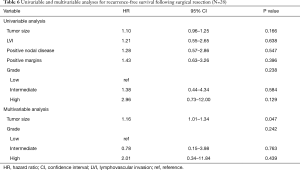
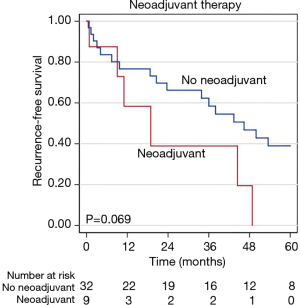
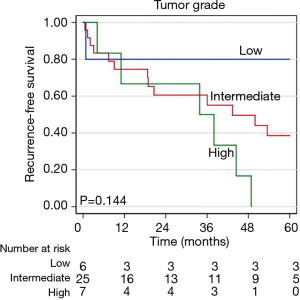
During a median follow-up time of 60.8 months following surgical resection (95% CI, 56.8–103.1) disease recurrence was observed in 29 (71%) patients, including 21 (51%) with intrathoracic recurrence. The median time to recurrence was 33.9 months (95% CI, 6.7–61.0 months). Pathologic variables were evaluated in univariable and multivariable analyses to determine predictors of recurrence after resection (Table 6). Tumor size and grade were included in a multivariable analysis for recurrence, whereupon tumor size was identified as a predictor of recurrence (P=0.047). Neither tumor grade, ACTH-production, nor the presence of LVI, positive nodal disease, or positive margins were found to be associated with disease recurrence. While not quite statistically significant, patients receiving neoadjuvant therapy appeared to have a poorer RFS (P=0.069), in an unadjusted Kaplan-Meier analysis for disease recurrence (Figure 2). Similarly, there appeared to be differential RFS trends based upon tumor grade (P=0.144) with those having high-grade tumors having the shortest RFS (Figure 3).
Full table
Discussion
While few case reports and case series exist which report the outcomes of patients treated for thymic NET, larger investigations are rare. Furthermore, though a report of the experience of 160 patients captured in the SEER database was previously reported, this inherently represents dissimilar patients and treatment methods. Differential survival has been described based upon various tumor and treatment factors, but investigations examining various treatment algorithms and predictors of outcomes have yet to be published. This study represents the largest single center experience presented to date, aiming to answer these important issues of predictors of survival following disparate management strategies.
In the present study, we aimed to share our experience in the management of thymic NET, as well as to describe patterns predicting OS and RFS. In doing so, we identified a heterogeneous population of tumors and treatment strategies. With respect to disease management, the majority of patients underwent surgical resection, which was associated with survival in unadjusted Kaplan-Meier analyses and was suggestive of benefit in Cox multivariable regression models. Furthermore, following resection, increasing tumor size was associated with development of recurrence. Therefore, surgical resection appears paramount to survival, and patients with larger tumors may benefit from adjunctive therapies to prevent future relapse of disease.
Prior literature has suggested that thymic NET represent tumors with variable histopathologic characteristics with a historically poor prognosis overall (3). Relative resistance to chemotherapeutic options has also been noted (14). However, more recent evidence from national and international databases has been suggestive of a more optimistic prognosis, which may, in part, be due to improvements in identification and classification of disease, in addition to the use of adjunctive therapies (2,10,12).
Specifically, we report herein a more than doubling of the 5-year survival as compared to earlier reports. However, our data are similar to those presented by Filosso et al., gathered from the International Thymic Malignancy Interest Group, as well as the European Society of Thoracic Surgeons (15). These authors reported the experience of over 200 patients, most of whom were found to have atypical carcinoid with a median OS of 7.5 years. The observed 5-year survival of this cohort was 68%, which exactly mirrors our observations, and was very strongly influenced by disease stage, as suggested by our Kaplan-Meier analysis.
Our findings also suggest a more aggressive treatment approach for those assessed to have advanced or high-grade disease. Specifically, while it appeared that those receiving neoadjuvant therapy prior to resection suffered earlier death, it is likely that induction therapy was used due to poorer prognosis upon presentation. These results are similar to a prior investigation which demonstrated poorer OS for patients receiving radiation therapy at any point during disease management (4).
While there were scattered patients with metastatic disease in our cohort, this represented a small proportion overall, which is an important distinction from previous literature. In fact, a majority of our cohort was composed of patients with early stage disease. This likely, at least in part, accounts for an apparent improvement in OS. As part of a report of the experiences of 160 patients with thymic NET gathered from the SEER database, Gaur et al reported a 5-year survival rate of 53% in all comers. However, unlike our investigation, however, 70% of this population had regional or distant disease at the time of diagnosis, as compared to 55% in our cohort. Furthermore, 68% of patients in their cohort were noted to have low- or intermediate-grade tumors, while a higher proportion (82%) of patients in our study had tumors of these classifications.
Additionally, while this disease may display a relatively aggressive tumor biology in comparison to benign thymoma, optimal classification of these tumors has been challenging, resulting in several unique staging systems, and recent investigations to identify biomarkers which may further describe the disease and guide management (16). Given these unique criteria, staging and treatment should be evaluated in the context of all proposed staging models and any available molecular biomarkers which may aid in treatment optimization. Differences in available staging classifications can be observed in our cohort, in which a small proportion of patients had M1 disease by TNM staging, though over half of the cohort had stage IV disease according to Masaoka-Koga classification, chiefly due to nodal metastatic disease. As such, evaluation of patients on a case-by-case basis in a multidisciplinary setting is paramount to treatment of this disorder.
Our study is not without limitations, which include its design as a retrospective review. Therein, the time frame over which patients were evaluated includes multiple editions of the WHO classifications of thymic NET, changes to treatment regimens especially chemotherapy, as well as an improvement in the understanding of the biology of this disease. Additionally, our findings represent the experience of a single tertiary referral center and, thus, may not be generalizable to the overall population or other facilities.
In conclusion, surgical resection is important to survival for patients presenting with thymic NET, and should be pursued when possible. Additionally, larger tumor size is associated with a shorter disease-free interval, and should be considered when evaluating a patient for adjuvant therapy. Our findings furthermore suggest that, in patients with aggressive or advanced disease, use of adjunctive therapies may not provide survival benefit.
Acknowledgments
The authors are indebted to the following individuals who were instrumental in identifying the individuals in our study cohort: Gregory Barbosa, Sireesha Murari, Edmond Jacobs, Emily Yu, and the IAI Support Team.
Footnote
Conflicts of Interest: This abstract was accepted for oral presentation at the Multidisciplinary Thoracic Cancers Symposium in San Diego, CA from March 14–16, 2019.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center (PA15-0742) with a waiver of informed consent.
References
- Lausi PO, Refai M, Filosso PL, et al. Thymic neuroendocrine tumors. Thorac Surg Clin 2014;24:327-32. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Chaer R, Massad MG, Evans A, et al. Primary neuroendocrine tumors of the thymus. Ann Thorac Surg 2002;74:1733-40. [Crossref] [PubMed]
- Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010;251:1117-21. [Crossref] [PubMed]
- Teh BT, McArdle J, Chan SP, et al. Clinicopathologic studies of thymic carcinoids in multiple endocrine neoplasia type 1. Medicine (Baltimore) 1997;76:21-9. [Crossref] [PubMed]
- Crona J, Bjorklund P, Welin S, et al. Treatment, prognostic markers and survival in thymic neuroendocrine tumours. a study from a single tertiary referral centre. Lung Cancer 2013;79:289-93. [Crossref] [PubMed]
- Wick MR, Rosai J. Neuroendocrine neoplasms of the thymus. Pathol Res Pract 1988;183:188-99. [Crossref] [PubMed]
- Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am J Clin Pathol 2000;114:100-10. [Crossref] [PubMed]
- Rosai J, Higa E. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor. Clinicopathologic study of 8 cases. Cancer 1972;29:1061-74. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: International Agency for Research on Cancer, 2015.
- Litvak A, Pietanza MC. Bronchial and Thymic Carcinoid Tumors. Hematol Oncol Clin North Am 2016;30:83-102. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Liu H, et al. Thymic neuroendocrine tumors (paraganglioma and carcinoid tumors): a comparative immunohistochemical study of 46 cases. Hum Pathol 2014;45:2463-70. [Crossref] [PubMed]
- Shewale JB, Nelson DB, Rice DC, et al. Natural History of Ground-Glass Lesions Among Patients With Previous Lung Cancer. Ann Thorac Surg 2018;105:1671-7. [Crossref] [PubMed]
- Yao JC. Neuroendocrine tumors. Molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract Res Clin Endocrinol Metab 2007;21:163-72. [Crossref] [PubMed]
- Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg 2015;149:103-9.e2. [Crossref] [PubMed]
- Dinter H, Bohnenberger H, Beck J, et al. Molecular Classification of Neuroendocrine Tumors of the Thymus. J Thorac Oncol 2019;14:1472-83. [Crossref] [PubMed]