
Advances in targeting acquired resistance mechanisms to epidermal growth factor receptor tyrosine kinase inhibitors
Introduction
Lung cancer remains the most common and deadliest malignancy worldwide. Incidence in 2018 has been reported to be 2.1 million new cases and mortality rates represent the highest for cancer-related disease at nearly 20% (1). The identification of epidermal growth factor receptor (EGFR) as an oncogenic driver and the subsequent development of EGFR-targeted therapy represent a revolutionary change in treatment for advanced non-small cell lung cancer (NSCLC).
Lung cancer was traditionally characterized as small cell or NSCLC with non-small cell histology further stratified into squamous, adenocarcinoma, and others (including large cell and neuroendocrine) representing 34%, 55%, and 11% of NSCLC, respectively (2). The distinction between squamous and adenocarcinoma has had predictive therapeutic implications, as pemetrexed has been shown to be preferentially effective in non-squamous histology (3). Application of histological subtype to predict therapeutic response was therefore an early indicator of “personalized” therapy. Now, the revolution and availability of sequencing technology has ushered in a new era of further sub-categorization of NSCLCs and targeted therapy against specific oncogenic driver mutations, with EGFR mutants serving as a successful model for drug development against a molecular target (2).
EGFR
EGFR mutations account for approximately 15% of all NSCLC cases. In Caucasian populations, this represents 10–20% of NSCLC compared to 30–40% in Eastern Asian populations (2,4,5). Prevalence is also significantly higher in females, non-smokers, and in adenocarcinoma histology (4).
EGFR is a membrane-bound receptor tyrosine kinase of the ErbB family. Activation causes downstream effects via several signaling pathways including RAS/MAPK, JAK/STAT, and PI3K/AKT/mTOR. Downstream effects include proliferation, migration, and survival (6,7). Activation of EGFR, therefore, is considered an oncogenic driver. EGFR mutants can be categorized as either activating mutations or resistance mutations.
EGFR activating mutations cause constituent activation of EGFR through ligand-independent dimerization and downstream signaling activation. Mutations of exons 18–21 are the most common, with nearly 90% due to deletions in exon 19 or a point mutation in exon 21 (L858R) (8). These mutants lend higher sensitivity to EGFR tyrosine kinase inhibitors (TKIs) owing to an open ATP-binding pocket and lower affinity for ATP itself, thus allowing a competing compound to bind instead (9,10). Other less common activating mutations include exon 20 insertions and mutations at G719X, L861Q, S768I, as well as compound heterozygous mutations in EGFR (8,11-13).
EGFR exon 20 insertions account for 5–12% of EGFR mutations in NSCLC (9,14). Although they are activating mutations, the mechanism of constituent activation of the tyrosine kinase is unique to that of deletion 19 or L858R. While exon 19 deletions and L858R are considered sensitizing mutations to TKI therapy, insertions of exon 20 are typically resistant to approved EGFR-TKIs with the uncommon exception of the proximal A763_Y764insFQEA mutant (15-18). Exon 20 insertion resistance to EGFR-TKI has been attributed to a conformational change resulting in steric hindrance in the ATP-binding pocket (9,19). Additional means of resistance include the conformational change that induces constituent activation without reducing ATP affinity or increasing affinity for 1st generation EGFR-TKIs (17).
In rare cases, germline mutations in EGFR have been reported that increase the risk of developing lung cancer, including the rarely reported de novo T790M mutation (20,21). This germline mutation itself can lead to EGFR-mutant NSCLC; however, the development of malignancy frequently co-occurs with a second EGFR mutation (20). Similar to T790M that arises as an acquired resistance mechanism, germline T790M may be sensitive to 3rd generation TKI (22).
Current guidelines for treatment of advanced non-small cell lung cancers (NSCLC) now include identification of EGFR mutations at baseline (23); however, most patients on therapy will develop resistance via acquired mutations. In addition to bypass tracts such as MET and HER2 amplification, secondary on-target resistance mutations while on therapy using EGFR-TKIs can develop. This includes on-target mutations to first-generation EGFR-TKI such as T790M or to third generation EGFR-TKI such as C797S. Multiple clinical trials are underway to evaluate safety and preliminary efficacy of therapeutic strategies to target and overcome EGFR-mutant NSCLC that has developed acquired resistance to currently approved EGFR-TKIs (Table 1).
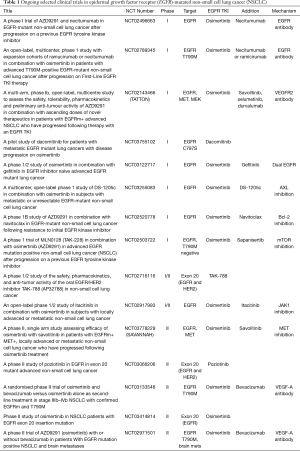
Full table
First-generation TKIs
First-generation, reversible tyrosine kinase inhibitors (TKI) include gefitinib and erlotinib which exert their effect by competing against ATP at an ATP-binding site (24). Clinical trials examining first-line gefitinib compared to standard chemotherapy has demonstrated significantly improved progression-free survival (PFS) and overall response rates (ORR) (25-27). Gefitinib versus chemotherapy in both NEJ002 and IPASS resulted in similar overall survival between treatment arms, although this was attributed to high crossover to gefitinib therapy in chemotherapy groups (28,29). First-line erlotinib has been examined compared to chemotherapy in the OPTIMAL, EURTAC, and ENSURE trials with findings of improved PFS and ORR, with equivalent overall survival (OS), again suggesting cross-over effect (30-33). Although response rates for first-generation TKIs are up to 70%, inevitably patients will progress after approximately 12 months (34,35).
Second-generation TKIs
Second generation TKIs include afatinib and dacomitinib which are irreversible EGFR-TKIs that covalently bond to C773 and C797 of EGFR, respectively (36,37). Afatinib additionally covalently binds to C805 of HER2 and has been suggested to have activity against T790M in pre-clinical studies (37), although this has not been demonstrated in clinical studies thus far because of its inability to achieve serum concentrations to effectively inhibit T790M in patients without substantial toxicity.
Afatinib in EGFR-mutated adenocarcinoma has been compared against chemotherapy in the LUX-Lung clinical trials. In both LUX-Lung 3 and 6, median PFS (mPFS) for afatinib was near 11 months compared to 6.9 months for cisplatin/pemetrexed and 5.6 months for gemcitabine/cisplatin (38,39). Follow-up OS analyses showed no difference between the afatinib and cisplatin-based chemotherapy regimens in all comers; however, a subset of patients with exon 19 deletions showed improved OS in pooled analysis of LUX-Lung 3 and 6. In exon 19 deletion patients, median OS for LUX-Lung 3 was 33.3 months in the afatinib group compared to 21.1 months in the chemotherapy group (HR 0.54, P=0.0015). Similar findings were seen in LUX-Lung 6, with median OS 31.4 months in the afatinib group compared to 18.4 months in the chemotherapy group (HR 0.64, P=0.023). This effect was not seen in L858R mutants (40). Afatinib has also been examined against placebo in patients that failed chemotherapy and first-generation TKIs. This demonstrated a median PFS benefit of 2 months but no OS benefit (41). LUX-Lung 7 compared afatinib to gefitinib, with a mild improvement in time to treatment failure of 2 months but with more toxicity, predominantly diarrhea and rash. Follow-up analyses demonstrated higher responses with afatinib (72.5% vs. 56.0%) but similar OS including exon 19 deletion and L858R subgroups (42).
Afatinib with cetuximab, a monoclonal antibody against EGFR that reduces the EGFR burden in tumors, has been examined in the setting of erlotinib and gefitinib resistance. Combination afatinib and cetuximab was studied in a phase Ib trial of patients with advanced EGFR-mutated NSCLC resistant to 1st generation TKIs (43). Response rates of 29% and median PFS of 4.7 months were similar regardless of acquired T790M status. The afatinib and cetuximab combination was furthermore examined in a sequential treatment strategy. Patients who progressed on 1st generation TKI, then progressed on afatinib were additionally given cetuximab (44). Overall response rate was 11% with median PFS 2.9 months. Results favored the T790M-positive tumors, as all responders were in this group and median PFS was 4.8 vs. 1.8 months for T790M-negative tumors. SWOG 1403 examined front-line afatinib with or without cetuximab in EGFR exon 19 deletion or L858R mutants; however, accrual was halted early owing to interim analysis demonstrating futility in PFS, OS, and time to treatment discontinuation (45). For EGFR exon 20 insertion patients, a small study of afatinib and cetuximab showed promise, as partial responses were seen in 3 out of 4 patients with median PFS of 5.4 months (46).
Dacomitinib has been less well-studied than afatinib but has also been evaluated in the phase III ARCHER 1050 trial, which lead to FDA approval in front-line therapy. Compared to gefitinib, dacomitinib resulted in higher median PFS (14.7 vs. 9.2 months), higher duration of response (14.8 vs. 8.3 months), but also higher rates of grade 3 and higher adverse events (63% vs. 41%) including diarrhea, paronychia, and rashes (47).
Uncommon EGFR mutations
Of the EGFR-mutated NSCLCs, exon 19 deletion and L858R accounts for the vast majority. Other less common mutants include exon 20 insertion, G719X, L861Q, S768I, and compound heterozygous mutations in EGFR. These represent 5–10% of EGFR mutants with exon 20 insertion being the most frequent of the uncommon mutations (12,13). Afatinib has demonstrated efficacy against G719X, L861Q, and S768I and is FDA approved for these mutations, although only 75 out of 600 patients in the study had uncommon EGFR mutations (48). A phase II trial of osimertinib in 35 patients with EGFR mutations other than exon 19 deletion, L858R, and exon 20 insertions has also suggested preliminary efficacy but this is not yet conclusive (49).
T790M
Invariably, patients develop resistance and progress on EGFR-TKI treatment. Identification of mechanisms of resistance to first- and second-generation EGFR-TKI were initially examined in case reports and small retrospective studies with a common finding of a secondary de novo EGFR mutation of exon 20 with methionine to threonine substitution at position 790 (T790M) (50-53). Threonine 790 has been considered a “gatekeeper” given its location in the ATP binding cleft (24,54). Therefore, one proposed mechanism of resistance for T790M has been steric hindrance (24) but further examination has shown that the mechanism is likely due to increasing ATP affinity, thus superseding the ATP-competing effects of first-generation TKIs (54).
Subsequent larger retrospective studies, prospective studies, and meta-analyses have shown approximately 50–60% of patients treated with first- or second-generation EGFR-TKI develop T790M mutation, with other less frequent mechanisms including secondary mutations bypassing the EGFR pathway (e.g., BRAF, PIK3CA, KRAS, MET amplification, HER2 or ERBB2 amplification), histologic transformation (e.g., small cell transformation), and other EGFR mutations (35,55-59). Amongst reports studies of paired pre- and post-progression tumor genetics that have analyzed T790M, HER2 amplification, MET amplification, or PIK3CA, overlap of multiple oncogenic drivers are commonly reported as a means of acquired resistance (Figure 1) (55,59-62).
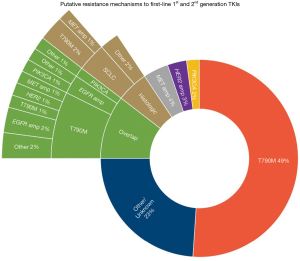
Although afatinib was promising against T790M in pre-clinical studies, subsequent clinical studies have shown rates of acquired T790M mutations comparable to first-generation TKIs (35,58,63). Dacomitinib long-term response is similarly limited by T790M and additionally C797S mutations (64).
Third-generation TKIs
Osimertinib is a third-generation EGFR-TKI that irreversibly binds to the EGFR-TKD and is able to overcome resistance mediated by EGFR-T790M. The mechanism of action is via covalent binding to the C797 residue of the ATP-binding domain of EGFR (65,66). Its effects are mechanistically unique from first and second-generation TKIs with additional activity against T790M mutants in addition to activity against canonical EGFR-sensitizing mutations (exon 19 deletion and L858R) while sparing wild-type EGFR more than previous generation EGFR-TKIs (66).
Osimertinib was examined in patients with EGFR-mutated NSCLC that failed first-generation TKIs with promising results (67). Sixty two percent of the enrolled patients in this study had T790M, with approximately three-fold higher response rates and median PFS compared to non-T790M cases (T790M positive—ORR 61%, mPFS 9.6 months; T790M negative—ORR 21%, mPFS 2.8 months). The phase I AURA trial initially demonstrated promising results with response rates over 70% and median PFS of 20 months (68). AURA3 furthermore examined osimertinib compared to platinum/pemetrexed chemotherapy in patients with progression after first-generation TKIs and T790M mutation (69). Osimertinib had higher response rates and median PFS compared to platinum-based chemotherapy (ORR 71% vs. 31%; mPFS 10.1 vs. 4.4 months; HR 0.30; 95% CI, 0.23–0.41; P<0.001). Median PFS benefit was also seen in patients with brain metastases (8.5 vs. 4.2 months; HR 0.32; 95% CI, 0.21–0.49; P value not reported).
The pivotal FLAURA trial examined front-line osimertinib against first-generation TKIs in EGFR-mutated patients with either exon 19 deletion or L858R (70). Compared to first-generation TKIs, osimertinib demonstrated comparable response rates but with significantly higher median PFS (18.9 vs. 10.2 months; HR 0.46; 95% CI, 0.37–0.57; P<0.001) and median duration of response (17.2 vs. 8.5 months). A trend towards improved OS was shown (18-month survival: 83% vs. 71%; HR 0.63; 95% CI, 0.45–0.88), though has not reached sufficient accrual yet to determine statistical significance. Toxicity profile also favored osimertinib. In patients with CNS metastases, the progression-free survival and response rates were similar to the overall population with progression-free survival still favoring osimertinib.
Rociletinib is a 3rd generation EGFR-TKI that covalently binds the C797 residue and at first showed promise in a phase I/II clinical trial TIGER-X of 130 patients (later enrolling over 600 patients) in the second-line setting (71,72). Median follow-up time was only 10.5 weeks and unconfirmed responses were included in analysis. ORRs were initially reported as 59% amongst T790M-patients. Updated results however showed ORRs of 45% (73). Side effects notably included QT prolongation (grade 3, 5%) and hyperglycemia (grade 3, 22%), the latter owing to a rociletinib metabolite inhibiting insulin growth factor receptor 1. Unfortunately, due to less-than-expected ORRs, side effects, and a better tolerated alternative in osimertinib, the FDA declined to grant rociletinib accelerated approval and its development was subsequently halted (72).
Similar to earlier generation TKIs, a similar problem of eventual progression and development of acquired resistance exists with 3rd generation TKIs. Examination of patients who progressed on previous generation EGFR-targeted therapy and then progression on osimertinib initially revealed three patterns of resistance: loss of T790M, maintenance of T790M with or without the EGFR C797S mutation (74). These findings have been confirmed in larger patient cohorts with the C797S mutation (75,76). Interestingly, loss of T790M may portend a worse prognosis as it has been associated with significantly shorter time to discontinuation of therapy (6.1 vs. 15.2 months) (75). Other resistance pathways have included EGFR L718 and L792 mutants and small cell transformation (74,75,77,78).
Putative resistance mechanisms of 1st line osimertinib
The phase I AURA trial examined osimertinib in sixty EGFR-mutated, NSCLC, treatment-naïve patients. Nineteen patients with progression underwent next-generation sequencing analysis of peripheral blood for identification of putative resistance mechanisms. In contrast to earlier generation EGFR-TKIs, acquired T790M was not identified as a resistance mechanism. Of these cases, other resistance mutations included C797S, MET amplification, and KRAS, PIK3CA, HER2, and JAK2 mutations (68).
Preliminary data from the FLAURA trial has resulted in unique resistance patterns for front-line osimertinib use compared to second-line osimertinib use (Figure 2). Resistance mechanisms included MET amplification (15%), C797S mutation (7%), and less commonly HER2 amplification, PIK3CA mutation, and RAS mutations (79). T790M was not detected as a secondary mutation as it is suppressed by osimertinib and unable able to develop without exposure to prior first- or second-generation EGFR-TKI. This is in stark contrast to standard-of-care 1st generation EGFR-TKI which demonstrates T790M as the most common resistance mechanism, seen in over half of these patients. As understanding of resistance mechanisms to 1st line osimertinib has evolved, so have efforts to identify combinatorial strategies to overcome these resistant EGFR-mutant NSCLCs (Table 1).

The landscape of resistance mechanisms to targeted therapy continues to evolve with the increasing availability and cost-effectiveness of next-generation sequencing, whether using peripheral blood or tissue samples. The current practice model of identifying oncogenic drivers and development of targeted therapies against these mutations has translated into clinical success. Unfortunately, this strategy does not account for other concurrent mutations and is limited by the development of resistance mechanisms. TKI therapy seemingly applies selective pressures to increase genomic complexity (80). Furthermore, development of resistance has been associated with higher mutational burden and worse prognosis (81). This highlights the importance of identifying pathways to resistance and subsequent therapies to either prevent or directly treat resistant clones.
On target EGFR-TKI resistance mechanisms
C797S
The C797S mutation is a substitution of cysteine at position 797 of EGFR exon 20 for serine, thus altering the binding site of osimertinib and mechanistically mediating resistance. This mutant accounts for 20–25% of resistance to osimertinib when used as second-line or later therapy (75,76). In vitro experiments have demonstrated allelic variation for the acquisition of C797S, as the mutant can occur either on the same (cis) or different (trans) allele with respect to T790M. Cis mutants with C797S and T790M have been suggested to be more resistant to EGFR-directed therapy including combination first and third generation TKIs whereas trans mutants retain sensitivity to combination TKI therapy (77). This approach has been attempted in the clinical setting where identification of patients with trans T790M and C797S mutants have had brief success with combination osimertinib and first-generation TKI (82,83). Development of cis mutation may also play a role in acquired resistance to combination therapy in initially trans mutants (83). Amongst C797S-mediated resistance to third-generation TKI, cis mutants seem to be much more common than trans mutants (84).
In front-line therapy with third generation TKI, C797S develops in the absence of T790M (79). This is a unique scenario that includes those with activating EGFR mutations (i.e., either exon 19 deletion or L858R) and C797S without T790M. In vitro experiments have suggested sensitivity to first generation TKIs in this setting whereas the addition of T790M confers additional resistance (77).
EGFR T790M/C797S
The so-called triple mutant of activating EGFR mutation, T790M, and C797S has been a challenging scenario to treat especially in the setting of cis mutants. A pre-clinical study of triple-mutated T790M, C797S, and exon 19 deletion has suggested synergistic efficacy for combination brigatinib, an anaplastic lymphoma kinase inhibitor with additional activity against EGFR, and cetuximab, an antibody against EGFR (85). Combination therapy has been tested pre-clinically in L858R triple mutants as well (86). EGFR allosteric inhibitors of the EGFR ATP-binding site such as EAI045 have been postulated to have activity in this setting, as the L858R mutation helps enlarge the allosteric binding site. Cetuximab further exposes this allosteric binding site through repositioning and disruption of the EGFR dimer, which has been observed in pre-clinical models of L858R triple mutants (86). Combination EAI045 and cetuximab in both double-mutated (L858R, T790M) and triple-mutated (L858R, T790M, C797S) offered synergistic efficacy in the mouse model (87). Whether these strategies translate into successful therapy in the clinical setting is yet to be known.
MET aberrations (amplification and mutation)
The mesenchymal epithelial transition (MET) factor is a proto-oncogene encoding for a receptor tyrosine kinase c-MET whose ligand is hepatocyte growth factor (HGF). Activation results in downstream signaling effects via MAPK, PI3K, SRC, and STAT pathways (88,89). Shared downstream effects contribute to synergism between MET and EGFR on oncogenesis (90). MET alterations, specifically amplification, have been identified as a driver mutation in a variety of malignancies including lung cancer, and are typically associated with a poor prognosis (91). MET mutations have also been described as a resistance mechanism to third generation EGFR-TKI albeit at a lower frequency that MET amplification (76). MET amplification occurs as a resistance mechanism though all generations of EGFR-TKI but appears to be more frequent for progression with osimertinib than previous generation EGFR-TKI (Figures 1,2).
MET amplification in the setting of first and second generation EGFR-TKI accounts for only 5% of acquired resistance (57). In front-line osimertinib, MET amplification accounts for nearly 15% of acquired resistance (79). Multiple trials are ongoing to examine the efficacy of combination EGFR-TKI with MET inhibitors, including the TATTON and SAVANNAH trials (Table 1). Preliminary data for the TATTON trial investigating savolitinib with osimertinib after progression on prior EGFR-TKI has shown promising preliminary results in c-MET positive patients, defined by FISH (amplification of MET/CEP7 ratio ≥2 or polysomy with copy number ≥5), NGS (≥20% tumor cells, ≥200× sequencing depth of coverage, and ≥5 copies over tumor ploidy), or IHC (staining 3+ in ≥50% of tumor cells). In patients with prior 1st or 2nd generation EGFR-TKI, objective response rate was 52% with a stable disease rate of 35%. In patients with prior 3rd generation EGFR-TKI, objective response rate was 25% with a stable disease rate of 44%. Most common grade ≥3 AEs included rash, transaminitis, and fatigue (92,93).
HER2
HER2 or ErbB2 is another receptor tyrosine kinase but without a known ligand. HER2 overexpression and amplification biases the receptor equilibrium towards dimerization, either homodimers or heterodimers with other ErbB receptors. This triggers downstream signaling through pathways such as PI3K and RAS/MAPK that ultimately promote cell survival and proliferation (94). HER2 overexpression is most well-known for its role in breast cancer oncogenesis, and targeted antibody therapy against HER2 has been critical to the success of treating breast cancer. HER2 mutations appear to be a distinct mechanism of HER2 activation and oncogenesis compared to HER2 gene overexpression/amplification, and the two entities rarely overlap (95). HER2 overexpression/amplification represents approximately 12% of acquired resistance to previous generation EGFR-TKI (96). This is in contrast to 1st line osimertinib where the frequency of HER2 overexpression/amplification is closer to 2% although this may be underestimated due to detection with plasma ctDNA (79).
HER2 is targeted through either small molecular inhibitors or antibodies, and clinical efficacy has been mixed in NSCLC. HER2-directed antibody therapy such as trastuzumab is a well-known success story in HER2-overexpressed breast cancers; however, this success has not translated to lung cancer based on a prior phase II trial in the second-line setting after chemotherapy (97). In cases with EGFR mutations, HER2 overexpression, and progression on prior EGFR-TKI, the combination of trastuzumab and paclitaxel has shown modest clinical efficacy with objective response rate of 46% and median duration of response 5.6 months (95% CI, 3.8–7.3) (98).
Afatinib is a 2nd generation EGFR-TKI with HER2 activity and may have activity in HER2 exon 20 mutated NSCLC based on a small exploratory phase II study (99). Osimertinib has also been suggested to have additional activity against HER2 (100). In in vivo mouse models, osimertinib was effective against HER2-mediated resistance in EGFR exon 19 deletion with HER2 overexpression, but not against exon 20 insertion HER2 mutants. Notably, the addition of a BET inhibitor may have a synergistic effect via enhanced pro-apoptotic signaling when combined with osimertinib (100).
Fusion bypass tracts
In addition to resistance via C797S, MET amplification, and HER2 overexpression in osimertinib therapy, fusion mutants have also been described as a mechanism of acquired resistance. This commonly includes fusions with BRAF or RET (101). Cell lines of PCPB2-BRAF fusions were found to be sensitive to trametinib, a MEK inhibitor, but not BRAF inhibitors. In a CCDC6-RET fusion cell line model, combination osimertinib and BLU-667, a RET inhibitor, was found to cause lower levels of downstream ERK and AKT phosphorylation while also reducing cell viability. This strategy of combinatorial osimertinib and BLU-667 was utilized in two patients with RET fusions and demonstrated encouraging responses (101).
EGFR exon 20 insertions
EGFR exon 20 insertions represent the third most common set of EGFR activating mutations and are considered “insensitive” activating mutations owing to a lack of response to 1st generation EGFR-TKIs, except for the proximal A763_Y764insFQEA variant (17). The mechanism for resistance is multi-factorial including steric hindrance, conformational change “locking” EGFR in an activated state, unchanged ATP-binding affinity, and unchanged EGFR-TKI affinity compared to wild type EGFR (9).
Poziotinib is a small, selective, and flexible small molecular inhibitor of EGFR and HER2 that can bypass effects of steric hindrance. Poziotinib has shown efficacy in pre-clinical models including patient-derived xenografts and mouse models of EGFR and HER2 exon 20-mutated NSCLC. This treatment strategy was then evaluated in a small phase II trial of EGFR exon 20-mutated NSCLC (19,102) where updated analysis included 40 evaluable patients. Starting dose was set at 16 mg but 45% of patients required reduction to 12 mg or less due to toxicity. Most common grade ≥3 adverse events were rash and diarrhea. Objective response at 8 weeks was 58% (95% CI, 40.9–73.0%), disease control rate was 90% (95% CI, 76.3–97.2%), and median PFS was 5.6 months (95% CI 5.06–NA months).
TAK-788 is a small molecular inhibitor of EGFR and HER2 with activity against EGFR exon 20 insertions (103). In a phase II trial of 34 patients, 62% of patients harbored EGFR exon 20 insertions. Fourteen patients were evaluable for response, of which three patients had partial response and six patients had stable disease. Notably, all patients with partial responses had EGFR exon 20 insertions. Most common AEs included diarrhea, nausea, fatigue and serious AEs included pulmonary toxicity (103).
TAS6417 is a TKI that binds to the ATP-binding pocket of EGFR with activity against exon 20 insertion and is EGFR wild-type sparing. This selective EGFR-TKI has shown promise in pre-clinical cell line studies demonstrating reduced levels of EGFR phosphorylation and downstream signaling markers such as AKT and ERK while showing increased levels of apoptotic markers. Tumor response has been seen in both in vitro and in vivo PDX models (104).
Osimertinib is a front-line option in T790M-mutated NSCLC; however, it has additional activity against exon 20 insertion mutants. This effect was first seen in in vitro cell line experiments (105). Subsequent examination of EGFR exon 20 insertion in cell-line and mouse xenograft studies have supported osimertinib and its metabolite AZ5104 as a viable therapy option. Furthermore, osimertinib and AZ5104 were more efficacious for tumor growth inhibition than afatinib (106). For HER2 exon 20 insertions, osimertinib has shown limited success in mouse models, although this may be overcome by combination with BET-inhibition (100). Adding an EGFR-monoclonal antibody (necitumumab) to osimertinib may also have activity against select EGFR exon 20 insertions (107). In another study, six Chinese patients with EGFR exon 20 insertions were treated with osimertinib; this resulted in a median PFS 6.2 months (95% CI, 5.0–12.9) over a median follow-up time of 6.2 months (108).
Anexelekto (AXL)
AXL is a receptor tyrosine kinase that is a member of the TAM (TYRO3, AXL, MERTK) family. Activation occurs via both GAS6 ligand-dependent and GAS6 ligand-independent mechanisms. AXL activation causes homodimerization, phosphorylation, and subsequently activation of multiple downstream pathways including PI3K, MEK, NF-κB, and JAK-STAT. This ultimately leads to proliferation, migration, and stemness. AXL activation also suppresses inflammation through inhibiting toll-like receptor responses, T-cell activation, NK-cell activity, and cytokine release (109). AXL and its ligand GAS6 as oncogenic drivers have been reported in a variety of malignancies, including lung cancer, and usually portends a poor prognosis and advanced disease (110). AXL expression has furthermore been implicated as a marker of resistance to multiple types of therapy, including chemotherapy and targeted therapy (109-111). This effect is additionally noted in EGFR NSCLC treated with osimertinib (112).
AXL expression frequency in NSCLC has been variably reported to be between approximately 30% to upwards of 90% with some differences likely attributable to inconsistent measurement modalities as AXL is typically evaluated using IHC (111). Its role in EGFR-mutated NSCLC has been investigated in preclinical and clinical studies. Furthermore, AXL expression is correlated with expression of other markers (e.g., vimentin) involved in epithelial-mesenchymal transition (EMT), suggesting transformation as a potential mechanism of AXL-mediated resistance (113). Mice xenograft models of EGFR-mutated NSCLC have shown AXL and GAS6 are associated with acquired resistance to erlotinib. In in vitro and in vivo models, EGFR-TKI sensitivity was restored with AXL inhibition, either genetically or pharmacologically with AXL-targeted antibodies or small molecular inhibitors. EGFR-TKI sensitive lines were induced to become EGFR-TKI resistant with overexpression of wild-type AXL as well. Therefore, AXL has been implicated to be both necessary and sufficient for erlotinib resistance (113). Similar results have been seen in studies of gefitinib-resistant EGFR-mutated in vitro models where AXL knockout restored gefitinib sensitivity, and AXL overexpression promoted gefitinib resistance (114).
The role of AXL in EGFR-TKI resistance has additionally been examined in patient tumors. In 35 patients with EGFR-mutated NSCLC patients progressive on 1st generation EGFR-TKIs, AXL and GAS6 expression by IHC were examined in addition to other mechanisms of resistance. Identified resistant mechanisms included T790M (29%), MET amplification (19%), GAS6 (25%), AXL (20%), and vimentin as a surrogate for EMT (20%). Three patients with baseline AXL mutations developed GAS6 overexpression after progression on EGFR-TKI (113). In a study of 26 Korean patients, a similar frequency of AXL mutants (19.2%) in EGFR-TKI-resistant NSCLC was seen (115).
AXL has been implicated in resistance to 3rd generation EGFR-TKI (112,116). Preclinical studies have suggested that osimertinib reduces expression of SPRY4, which suppresses AXL phosphorylation, thus leading to AXL activation and subsequent HER3, MET, and EGFR activation (112). Knockdown of 2 out of 3 of HER3, AXL, or EGFR led to synergistic reductions of cell viability (112). AXL and GAS6 expression have been inversely correlated to 1st and 3rd generation EGFR-TKI susceptibility (112,116). Decreasing AXL expression in PDX models and in vitro models restores osimertinib sensitivity through suppression of AKT signaling, and combining AXL inhibition and osimertinib may prevent emergence of resistant clones (112,116).
Histologic transformation
Transformation of EGFR-mutated NSCLC represents an alternative means of acquired resistance that often necessitates treatment with chemotherapy. Although next-generation sequencing represents a comprehensive method of identifying genomic changes, it is unable to detect histological changes especially when blood-based analysis is used. The most common transformation is to small cell lung cancer, although epithelial-mesenchymal transitions and sarcomatoid transformations are also seen (55,60,62,117). These tumors typically maintain their founder EGFR mutant; however, in T790M disease this often is lost after transformation. Recurrent mutations such as p53, Rb1, and PIK3CA may be genomic clues indicative of transformation if histological examination is unavailable (117). AXL-mediated EMT has also been suggested to promote histologic transformation (113).
Aurora kinase A (AKA)
AKA is a serine/threonine kinase that regulates cell cycle progression, mitosis, and meiosis by controlling bipolar spindle assembly and chromosome separation (118). Overexpression effects include genomic instability, cell-cycle dysregulation, de-differentiation and ultimately tumorigenesis (118,119). In NSCLC, EGFR therapy may cause upregulation of TPX2, an upstream activator of AKA. Activation of AKA causes apoptotic escape, mitotic abnormalities, and persistence and heterogeneity of EGFR-TKI-resistant clones (120). The addition of an AKA inhibitor, MLN8237, to 3rd generation TKI in in vitro and in vivo cell models has increased apoptosis, enhanced magnitude of response, and delayed emergence of resistance. Furthermore, AKA is likely necessary for survival of 3rd generation TKI-resistant clones as MLN8237 demonstrated activity when added sequentially after development of resistance (120).
Immunotherapy in EGFR-mutant NSCLC
EGFR-TKIs are standard-of-care first-line therapy options for EGFR-mutated NSCLC. Therefore, many immunotherapy clinical trials have excluded such tumors. Meta-analyses of clinical trials that have included EGFR mutants have shown that single-agent immune checkpoint inhibition with PD-1/PD-L1 antibodies does not provide an overall survival benefit in EGFR-mutated disease (121,122). This is likely due to lack of mutational load and smoking associated signature that relates to an increase in neoantigen specific T-cell activity (123). Interestingly, EGFR-mutant NSCLC with increased tumor mutational burden has been suggested to have inferior survival and earlier time to treatment discontinuation (81).
The combination of immunotherapy with EGFR-TKIs has generally not been successful owing to increased toxicities. Several clinical trials have examined this therapeutic strategy. Erlotinib with atezolizumab has been evaluated in a phase Ib study of 28 patients, 43% of whom had grade 3 adverse events most commonly pyrexia, rash, diarrhea, and transaminitis, with serious adverse events reported in half of all patients (124). Erlotinib with nivolumab was evaluated in a phase I study of 21 patients, and grade 3 events including transaminitis, diarrhea, and weight loss were seen in 23.8% of patients although no grade 4 or 5 toxicities were noted (125). Gefitinib with durvalumab has been evaluated in a phase I expansion trial with two arms: concurrent together (n=10), or gefitinib alone for 4 weeks followed by concurrent therapy (n=10). While response rates were approximately 80% amongst both arms, therapy was discontinued owing to transaminitis in 3 patients and pneumonitis in 1 patient—all from the second arm (126). The TATTON phase Ib trial explored osimertinib with durvalumab but unfortunately 38% of patients developed interstitial lung disease, whereas monotherapy with either alone resulted in only 2–3% of patients developing interstitial lung disease. This safety issue has halted this arm of the TATTON trial (127). The phase III CAURAL trial attempted to investigate osimertinib with durvalumab but was similarly stopped early due to concern of lung disease based on the TATTON trial (128).
Angiogenesis
Vascular endothelial growth factor (VEGF) is known for its role in angiogenesis; however, it has been implicated to have additional function in creating an immune-tolerant tumor microenvironment (129). Therefore, anti-VEGF therapy may synergistically enhance immunotherapy efficacy. On this basis, IMpower 150 sought to evaluate the efficacy of first-line atezolizumab, bevacizumab, and chemotherapy (carboplatin and paclitaxel) in metastatic non-squamous NSCLC, including in a subset of patients with EGFR and ALK mutations (130). Patients were randomized between three arms: (I) atezolizumab and chemotherapy followed by atezolizumab maintenance, (II) atezolizumab, bevacizumab, and chemotherapy followed by atezolizumab and bevacizumab maintenance, (III) bevacizumab and chemotherapy followed by bevacizumab maintenance. Data for the PD-L1, anti-VEGF, and chemotherapy arm compared to the anti-VEGF and chemotherapy arm have been reported. The former was associated with longer median PFS in the wild-type (8.3 vs. 6.8 months; HR 0.62; 95% CI, 0.52–0.74; P<0.001) and intention-to-treat populations (8.3 vs. 6.8 months; HR 0.61; 95% CI, 0.52–0.72). A longer median OS was observed in the wild-type population (19.2 vs. 14.7 months; HR 0.78; 95% CI, 0.64–0.96; P=0.02). Furthermore, median PFS was longer in the subset of patients with a higher T-cell gene expression signature (11.3 vs. 6.8 months; HR 0.51; 95% CI, 0.38–0.68; P<0.001). The subset of patients with EGFR or ALK mutations also seemingly derived median PFS benefit (9.7 vs. 6.1 months; HR 0.59; 95% CI, 0.37–0.94), although this was demonstrated in post-hoc analysis and included only 13.5% of the intention-to-treat population. Such a strategy of VEGF and PD-1 directed therapy would benefit from further validation in prospective randomized clinical trials in NSCLC with driver mutations.
The role of combinatorial VEGF inhibition and erlotinib has been examined in the NEJ026 trial. Patients were randomized to erlotinib plus bevacizumab 15 mg/kg every 21 days versus erlotinib monotherapy. One hundred twelve patients in each arm were evaluable for efficacy and safety at interim analysis. Median PFS for erlotinib and bevacizumab therapy vs. erlotinib monotherapy was 16.9 vs. 13.3 months, respectively (HR 0.605; 95% CI, 0.417–0.877; P=0.016). Grade 3 or higher adverse events were higher in the combinatorial arm (88% vs. 46%) and was mostly attributable to rash, with less common serious adverse events of neutropenia and liver dysfunction (131). The underlying mechanism of benefit for adding bevacizumab is unclear. It has been postulated to be due to the anti-angiogenesis and pro-apoptotic effects, although VEGF seems to have an additional role in immunomodulation of the tumor microenvironment (129).
Conclusions
The advent of next-generation sequencing and identification of driver mutations and their respective targeted therapies has revolutionized care of patients with metastatic lung cancer. While EGFR-TKIs are effective and safe therapies for EGFR-mutated NSCLC, survival is still limited by development of a diverse group of acquired resistance mechanisms that drive recurrent disease. We have discussed here the current landscape of resistance mechanisms to EGFR-targeted therapy within the context of contemporary treatment of EGFR-mutated NSCLC. As next-generation sequencing of both tissue and peripheral blood increases in clinical practice, our understanding of this process will evolve. There is a clear and present need to develop treatment strategies to either prevent or directly treat acquired resistance to next generation EGFR-TKI.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Trever G. Bivona) for the series “Mechanisms of Resistance to EGFR-targeted Therapy” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2019.08.32). The series “Mechanisms of Resistance to EGFR-targeted Therapy” was commissioned by the editorial office without any funding or sponsorship. JWR reports personal fees from Celgene, personal fees from Heron, personal fees from Takeda, personal fees from Abbvie, grants and personal fees from Boehringer Ingelheim, personal fees from Loxo Oncology, personal fees from Spectrum, personal fees from Medtronic, grants from Merck, grants from Novartis, grants from AstraZeneca, outside the submitted work. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Li T, Kung HJ, Mack PC, et al. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013;31:1039-49. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 2016;7:78985-93. [Crossref] [PubMed]
- Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a sys-tematic review and global map by ethnicity (mutMapII). Am J Cancer Res 2015;5:2892-911. [PubMed]
- Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B 2015;5:390-401. [Crossref] [PubMed]
- Yewale C, Baradia D, Vhora I, et al. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials 2013;34:8690-707. [Crossref] [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [Crossref] [PubMed]
- Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther 2019;4:5. [Crossref] [PubMed]
- Eck MJ, Yun CH. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer. Biochim Biophys Acta 2010;1804:559-66. [Crossref] [PubMed]
- Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 2009;28 Suppl 1:S24-31. [Crossref] [PubMed]
- O'Kane GM, Bradbury PA, Feld R, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer 2017;109:137-44. [Crossref] [PubMed]
- Kate S, Chougule A, Joshi A, et al. Outcome of uncommon EGFR mutation positive newly diagnosed advanced non-small cell lung cancer patients: a single center retrospective analysis. Lung Cancer (Auckl) 2019;10:1-10. [Crossref] [PubMed]
- Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J Thorac Oncol 2018;13:1560-8. [Crossref] [PubMed]
- Gergis C, Rangachari D, Fujii M, et al. EGFR-A763_Y764insFQEA: A unique exon 20 insertion mutation that displays sensitivity to all classes of approved lung cancer EGFR tyrosine kinase inhibitors. J Thorac Oncol 2019;37:e20593.
- Voon PJ, Tsui DW, Rosenfeld N, et al. EGFR exon 20 insertion A763-Y764insFQEA and response to erlotinib--Letter. Mol Cancer Ther 2013;12:2614-5. [Crossref] [PubMed]
- Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013;5:216ra177. [Crossref] [PubMed]
- Naidoo J, Sima CS, Rodriguez K, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: Clin-ical outcomes and response to erlotinib. Cancer 2015;121:3212-20. [Crossref] [PubMed]
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Gazdar A, Robinson L, Oliver D, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J Thorac Oncol 2014;9:456-63. [Crossref] [PubMed]
- Yu HA, Arcila ME, Fleischut MH, et al. Germline EGFR T790M Mutation Found in Multiple Members of a Familial Cohort. J Thorac Oncol 2014;9:554-8. [Crossref] [PubMed]
- Ancevski Hunter K, Friedland DM, Villaruz LC, et al. First-Line Osimertinib in Patients with Treatment-Naive Somatic or Germline EGFR T790M-Mutant Metastatic NSCLC. J Thorac Oncol 2018;13:e3-5. [Crossref] [PubMed]
- Hanna N, Johnson D, Temin S, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- Kumar A, Petri ET, Halmos B, et al. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol 2008;26:1742-51. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbour-ing mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR muta-tion-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:839-44. [Crossref] [PubMed]
- Lin YT, Chen JS, Liao WY, et al. Clinical outcomes and secondary epidermal growth factor receptor (EGFR) T790M mutation among first-line gefitinib, erlotinib and afatinib-treated non-small cell lung cancer patients with activating EGFR mutations. Int J Cancer 2019;144:2887-96. [Crossref] [PubMed]
- Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 2007;67:11924-32. [Crossref] [PubMed]
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702-11. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung ade-nocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Janjigian YY, Smit EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 2014;4:1036-45. [Crossref] [PubMed]
- Horn L, Gettinger S, Camidge DR, et al. Continued use of afatinib with the addition of cetuximab after progression on afatinib in patients with EGFR mutation-positive non-small-cell lung cancer and acquired resistance to gefitinib or erlotinib. Lung Cancer 2017;113:51-8. [Crossref] [PubMed]
- Goldberg S, Redman M, Lilenbaum R, et al. Afatinib With or Without Cetuximab for EGFR-Mutant Non-Small Cell Lung Cancer: Safety and Efficacy Results from SWOG S1403. J Thorac Oncol 2018;13:S343-4. [Crossref]
- van Veggel B, de Langen AJ, Hashemi SMS, et al. Afatinib and Cetuximab in Four Patients With EGFR Exon 20 Insertion-Positive Advanced NSCLC. J Thorac Oncol 2018;13:1222-6. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Cho JH, Sun J, Lee S, et al. An Open-Label, Multicenter, Phase II Single Arm Trial of Osimertinib in NSCLC Patients with Un-common EGFR Mutation(KCSG-LU15-09). J Thorac Oncol 2018;13:S344-S.
- Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res 2006;12:5764-9. [Crossref] [PubMed]
- Ma C, Wei S, Song Y. T790M and acquired resistance of EGFR TKI: a literature review of clinical reports. J Thorac Dis 2011;3:10-8. [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [Crossref] [PubMed]
- Ercan D, Zejnullahu K, Yonesaka K, et al. Amplification of EGFR T790M causes resistance to an irreversible EGFR inhibitor. Oncogene 2010;29:2346-56. [Crossref] [PubMed]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Wang ZF, Ren SX, Li W, et al. Frequency of the acquired resistant mutation T790 M in non-small cell lung cancer patients with active exon 19Del and exon 21 L858R: a systematic review and meta-analysis. BMC Cancer 2018;18:148. [Crossref] [PubMed]
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29:i10-9. [Crossref] [PubMed]
- Kaburagi T, Kiyoshima M, Nawa T, et al. Acquired EGFR T790M Mutation After Relapse Following EGFR-TKI Therapy: A Popula-tion-based Multi-institutional Study. Anticancer Res 2018;38:3145-50. [PubMed]
- Yu HA, Suzawa K, Jordan E, et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Ki-nase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin Cancer Res 2018;24:3108-18. [Crossref] [PubMed]
- Campo M, Gerber D, Gainor JF, et al. Acquired Resistance to First-Line Afatinib and the Challenges of Prearranged Progression Biopsies. J Thorac Oncol 2016;11:2022-6. [Crossref] [PubMed]
- Nakamura T, Nakashima C, Komiya K, et al. Mechanisms of acquired resistance to afatinib clarified with liquid biopsy. PLoS One 2018;13:e0209384. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Wu SG, Liu YN, Tsai MF, et al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget 2016;7:12404-13. [Crossref] [PubMed]
- Kobayashi Y, Fujino T, Nishino M, et al. EGFR T790M and C797S Mutations as Mechanisms of Acquired Resistance to Dacomitinib. J Thorac Oncol 2018;13:727-31. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Finlay MR, Anderton M, Ashton S, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem 2014;57:8249-67. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841-9. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700-9. [Crossref] [PubMed]
- Van Der Steen N, Caparello C, Rolfo C, et al. New developments in the management of non-small-cell lung cancer, focus on rociletinib: what went wrong? Onco Targets Ther 2016;9:6065-74. [Crossref] [PubMed]
- Sequist LV, Soria JC, Camidge DR. Update to Rociletinib Data with the RECIST Confirmed Response Rate. N Engl J Med 2016;374:2296-7. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4:1527-34. [Crossref] [PubMed]
- Yang Z, Yang N, Ou Q, et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2018;24:3097-107. [Crossref] [PubMed]
- Niederst MJ, Hu HC, Mulvey HE, et al. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin Cancer Res 2015;21:3924-33. [Crossref] [PubMed]
- Wang S, Tsui ST, Liu C, et al. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J Hematol Oncol 2016;9:59. [Crossref] [PubMed]
- Ramalingam SS, Rukazenkov Y, Todd A, et al. LBA50Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol 2018;29. [Crossref]
- Blakely CM, Watkins TBK, Wu W, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet 2017;49:1693-704. [Crossref] [PubMed]
- Offin M, Rizvi H, Tenet M, et al. Tumor Mutation Burden and Efficacy of EGFR-Tyrosine Kinase Inhibitors in Patients with EGFR-Mutant Lung Cancers. Clin Cancer Res 2019;25:1063-9. [Crossref] [PubMed]
- Arulananda S, Do H, Musafer A, et al. Combination Osimertinib and Gefitinib in C797S and T790M EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1728-32. [Crossref] [PubMed]
- Wang Z, Yang JJ, Huang J, et al. Lung Adenocarcinoma Harboring EGFR T790M and In Trans C797S Responds to Combination Therapy of First- and Third-Generation EGFR TKIs and Shifts Allelic Configuration at Resistance. J Thorac Oncol 2017;12:1723-7. [Crossref] [PubMed]
- Goldberg ME, Montesion M, Young L, et al. Multiple configurations of EGFR exon 20 resistance mutations after first- and third-generation EGFR TKI treatment affect treatment options in NSCLC. PLoS One 2018;13:e0208097. [Crossref] [PubMed]
- Uchibori K, Inase N, Araki M, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun 2017;8:14768. [Crossref] [PubMed]
- Ercan D, Choi HG, Yun CH, et al. EGFR Mutations and Resistance to Irreversible Pyrimidine-Based EGFR Inhibitors. Clin Cancer Res 2015;21:3913-23. [Crossref] [PubMed]
- Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016;534:129-32. [Crossref] [PubMed]
- Ma PC, Maulik G, Christensen J, et al. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev 2003;22:309-25. [Crossref] [PubMed]
- Kawakami H, Okamoto I, Okamoto W, et al. Targeting MET Amplification as a New Oncogenic Driver. Cancers (Basel) 2014;6:1540-52. [Crossref] [PubMed]
- Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog 2008;7:9. [Crossref] [PubMed]
- Scagliotti GV, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat Rev 2013;39:793-801. [Crossref] [PubMed]
- Yu H, Ahn M-J, Kim S-W, et al., editors. CT032 - TATTON Phase Ib expansion cohort: Osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-amplified NSCLC after progression on prior first/second-generation epidermal growth factor re-ceptor (EGFR) tyrosine kinase inhibitor (TKI). 2019 AACR Annual Meeting; 2019 March 31; Atlanta, GA.
- Sequist LV, Lee JS, Han JY, et al. CT033 - TATTON Phase Ib expansion cohort: Osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-amplified NSCLC after progression on prior third-generation epidermal growth factor receptor (EGFR) ty-rosine kinase inhibitor (TKI). 2019 AACR Annual Meeting; 2019 March 31; Atlanta, GA.
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127-37. [Crossref] [PubMed]
- Li BT, Ross DS, Aisner DL, et al. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J Thorac Oncol 2016;11:414-9. [Crossref] [PubMed]
- Takezawa K, Pirazzoli V, Arcila ME, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov 2012;2:922-33. [Crossref] [PubMed]
- Clamon G, Herndon J, Kern J, et al. Lack of trastuzumab activity in nonsmall cell lung carcinoma with overexpression of erb-B2: 39810: a phase II trial of Cancer and Leukemia Group B. Cancer 2005;103:1670-5. [Crossref] [PubMed]
- de Langen AJ, Jebbink M, Hashemi SMS, et al. Trastuzumab and paclitaxel in patients with EGFR mutated NSCLC that express HER2 after progression on EGFR TKI treatment. Br J Cancer 2018;119:558-64. [Crossref] [PubMed]
- De Grève J, Teugels E, Geers C, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 2012;76:123-7. [Crossref] [PubMed]
- Liu S, Li S, Hai J, et al. Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib. Clin Cancer Res 2018;24:2594-604. [Crossref] [PubMed]
- Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion. Cancer Discov 2018;8:1529-39. [Crossref] [PubMed]
- Heymach J, Negrao M, Robichaux J, et al. A Phase II Trial of Poziotinib in EGFR and HER2 exon 20 Mutant Non-Small Cell Lung Cancer (NSCLC). Journal of Thoracic Oncology 2018;13:S323-S4. [Crossref]
- Neal J, Doebele R, Riely G, et al. P1.13-44 Safety, PK, and Preliminary Antitumor Activity of the Oral EGFR/HER2 Exon 20 Inhib-itor TAK-788 in NSCLC. J Thorac Oncol 2018;13:S599. [Crossref]
- Hasako S, Terasaka M, Abe N, et al. TAS6417, A Novel EGFR Inhibitor Targeting Exon 20 Insertion Mutations. Mol Cancer Ther 2018;17:1648-58. [Crossref] [PubMed]
- Hirano T, Yasuda H, Tani T, et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget 2015;6:38789-803. [Crossref] [PubMed]
- Floc'h N, Martin MJ, Riess JW, et al. Antitumor Activity of Osimertinib, an Irreversible Mutant-Selective EGFR Tyrosine Kinase Inhibitor, in NSCLC Harboring EGFR Exon 20 Insertions. Mol Cancer Ther 2018;17:885-96. [Crossref] [PubMed]
- Riess JW, Groshen SG, Reckamp KL, et al. Osimertinib (Osi) plus necitumumab (Neci) in EGFR-mutant NSCLC: An ETCTN California cancer consortium phase I study. J Clin Oncol 2019;37:abstr 9057.
- Fang W, Huang Y, Hong S, et al. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer 2019;19:595. [Crossref] [PubMed]
- Gay CM, Balaji K, Byers LA. Giving AXL the axe: targeting AXL in human malignancy. Br J Cancer 2017;116:415-23. [Crossref] [PubMed]
- Rankin EB, Giaccia AJ. The Receptor Tyrosine Kinase AXL in Cancer Progression. Cancers (Basel) 2016;8:103. [Crossref] [PubMed]
- Zhang G, Wang M, Zhao HL, et al. Function of Axl receptor tyrosine kinase in non-small cell lung cancer Oncol Lett 2018;15:2726-34. (Review). [PubMed]
- Taniguchi H, Yamada T, Wang R, et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun 2019;10:259. [Crossref] [PubMed]
- Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012;44:852-60. [Crossref] [PubMed]
- Tian Y, Zhang Z, Miao L, et al. Anexelekto (AXL) Increases Resistance to EGFR-TKI and Activation of AKT and ERK1/2 in Non-Small Cell Lung Cancer Cells. Oncol Res 2016;24:295-303. [Crossref] [PubMed]
- Ji W, Choi CM, Rho JK, et al. Mechanisms of acquired resistance to EGFR-tyrosine kinase inhibitor in Korean patients with lung cancer. BMC Cancer 2013;13:606. [Crossref] [PubMed]
- Kim D, Bach DH, Fan YH, et al. AXL degradation in combination with EGFR-TKI can delay and overcome acquired resistance in human non-small cell lung cancer cells. Cell Death Dis 2019;10:361. [Crossref] [PubMed]
- Marcoux N, Gettinger SN, O'Kane G, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Oth-er Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol 2019;37:278. [Crossref] [PubMed]
- Damodaran AP, Vaufrey L, Gavard O, et al. Aurora A Kinase Is a Priority Pharmaceutical Target for the Treatment of Cancers. Trends Pharmacol Sci 2017;38:687-700. [Crossref] [PubMed]
- Lo Iacono M, Monica V, Saviozzi S, et al. Aurora Kinase A expression is associated with lung cancer histological-subtypes and with tumor de-differentiation. J Transl Med 2011;9:100. [Crossref] [PubMed]
- Shah KN, Bhatt R, Rotow J, et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat Med 2019;25:111-8. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Rudin C, Cervantes A, Dowlati A, et al. MA15.02 Long-Term Safety and Clinical Activity Results from a Phase Ib Study of Erlotinib Plus Atezolizumab in Advanced NSCLC. J Thorac Oncol 2018;13:S407. [Crossref]
- Gettinger S, Hellmann MD, Chow LQM, et al. Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC. J Thorac Oncol 2018;13:1363-72. [Crossref] [PubMed]
- Gibbons DL, Chow LQ, Kim DW, et al. Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 an-ti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naive patients (pts) with EGFR mutant NSCLC. J Thorac Oncol 2016;11:S79-S.
- Ahn MJ, Yang J, Yu H, et al. Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11:S115. [Crossref]
- Yang JC, Shepherd FA, Kim DW, et al. Osimertinib Plus Durvalumab versus Osimertinib Monotherapy in EGFR T790M-Positive NSCLC following Previous EGFR TKI Therapy: CAURAL Brief Report. J Thorac Oncol 2019;14:933-9. [Crossref] [PubMed]
- Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunothera-peutics. Semin Cancer Biol 2018;52:117-24. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019;20:625-35. [Crossref] [PubMed]


