Extensive abdominal and chest wall resection and reconstruction for invasive squamous cell carcinoma of the skin
Introduction
The effective incidence of cutaneous squamous cell carcinoma of the skin (cSCC) is unknown, in general cSCC is the second most common skin cancer, accounting for 20% of all cutaneous malignancies, with a continuous increase in its frequency (1). The skin of the thorax is a very rare localization for this kind of tumor (1-4). Despite cSCC is a malignant proliferation of keratinizing cells of the epidermis, generally, when the diagnosis is made, early surgical resection with adequate surgical margins is curative in more than 95% of the cases (5). Here we present a very rare case of local recurrence of a huge cSCC involving the anterior chest wall that underwent extensive chest wall resection associated to complex reconstruction by using a combination of synthetic materials and autologous flaps.
Surgical techniques
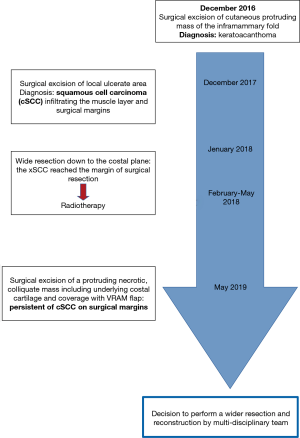
A 77-year-old man was admitted to our Institution with a 4-year history of multiple surgical attempts to cure a cSCC of the right anterior chest wall (Figure 1).
His oncological history began in 2016, when a cutaneous protruding mass appeared in the right inframammary fold and was subsequently excised, the diagnosis of keratoacanthoma was then made. As a complication of this procedure, the patient experienced wound suppurations that required multiple surgical enlargements of cutaneous margins.
One year later, in December 2017, the wound presented ulcerated and with an abundant purulent exudate. The plastic surgeons took the patient in charge and removed the ulcerated tissue lesion. Histological examination of the surgical specimen revealed a cSCC with infiltration of the muscular layer and of the surgical margins.
Because of this finding, in January 2018, he underwent a radicalization procedure that involved resection down to the costal plane, associated to the removal of the right V and VII costal cartilages and coverage of the defect with a lower abdominal flap. The lesion however reached the margins of the surgical resection; hence, he underwent adjuvant radiotherapy (60 Gy).
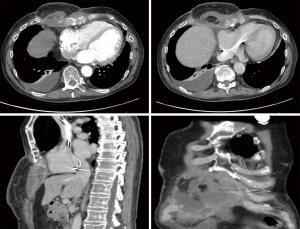
The patient remained in good clinical condition and without evidence of disease recurrence until March 2019, when a cutaneous secreting fistula appeared; he then gradually experienced worsening pain that required opioid therapy for its control. A computed tomography (CT) demonstrated the presence of a necrotic parasternal mass highly suspicious for a relapse of the malignancy.
Therefore, in May a new extensive radicalization was performed with removal of a large necrotic and colliquated mass, including also the anterior portion of underlying ribs and costal cartilages. The defect of the anterior thoracic wall was then covered by a left vertical rectus abdominis myocutaneous (VRAM) flap. The final pathology report demonstrated the persistence of cSCC on deep pre-sternal resection margins, the case was therefore discussed by a multidisciplinary team and it was agreed to perform a wider resection. Preoperative CT showed an extensive involvement by tumor of the xiphoid, lower body of the sternum and of the surrounding subcutaneous tissue. Moreover, multiple areas of necrosis and tissue colliquation were present at the level of the thoracic and abdominal wall with multiple fistulous tracts (Figure 2). The surgical procedure was carried out under general anesthesia and single lumen endotracheal tube ventilation. It required a multidisciplinary team of thoracic, abdominal and plastic surgeons. The previously VRAM flap was mobilized and the skin incision was extended to surround the neoplastic area up to the disease-free skin. Frozen sections examination of the cutaneous margins showed freedom from neoplastic involvement. The sternum was mobilized in its caudal part by resecting the sterno-chondral junction from the fourth to twelfth ribs bilaterally, then an en-bloc resection was performed by transecting the sternum about 2 cm below the angle of Louis. The remaining tumor involved the anterior part of the diaphragm and the hepatic dome, therefore, a tangential resection of the hepatic parenchyma and of the diaphragm was performed to reach macroscopically disease-free tissue. The reconstructive phase was carried out by using a combination of synthetic and autologous flaps. After placement of the chest tubes, a Gore-Tex® Dualmesh® (W. L. Gore, USA) prosthesis was put in place, anchored to the edges of the resected thoracic wall, to reconstruct both the lower anterior chest wall and the diaphragmatic dome. The prosthesis was fixed using U non-absorbable stiches. The prosthesis was covered with a previously prepared omental flap. The omentum is separated from the transverse colon end the great curvature of the stomach legating the gastroepiploic vessels. The right gastroepiploic vascular pedicle is identified and dissected mobilizing the omentum that after placemen of the synthetic mesh is rotated and fixed to cover it completely. Bilateral latissimi dorsi myocutaneous flaps were prepared by placing the patient in alternating lateral decubitus positions. The skin island is centered over the longitudinal coursing of latissimus dorsi muscle fibers. The anterior border of the cutaneous island is incised first, then the lateral border of the latissimus dorsi is retracted and the thoracodorsal neurovascular bundle is visualized and careful preserved. Now the skin incision is completed and the tendon of the muscle is divided. The muscle was tailored to fit with the wound to be filled. These two flaps were temporally placed in the axillary cavities and, by placing the patient in the supine decubitus, were positioned above the omental flap. Finally, the myocutaneous flaps and VRAM flap were sutured to cover the cutaneous thoracic defect. The remaining de-epithelialized areas were covered with mesh skin grafts taken from the right thigh (Figure 3). All surgical specimens were oriented and sent for final pathological examination that confirm an extensive infiltration of the tumor at the level of sternum, diaphragm, liver capsule and subcutaneous tissue. All the surgical margins resulted negative for tumor infiltration. The patient was extubated on post-operative date 3. The post-operative course was uneventful and the patient was discharged at home in 18 post-operative day. He continues medications of the surgical wound and skin grafts for following months until complete recovery. After six months from surgery the patient is free from disease recurrence.

Discussion
Surgery is the treatment of choice for cSCC and in most of the case is curative. The metastatic risk is very low: about 3–5%. It has been demonstrated that the rate of local recurrence is 4.6%, that the rate of lymph node involvement is 3.7% and that the cancer related mortality is 2.1% (1-5). The most important risk factor for local recurrence is obviously incomplete tumor removal (4,5). The maximal vertical tumor thickness, measured during histological examination, is another important risk factor for metastatic spread and local recurrence (4,5). The guidelines suggest that surgical resection margins should be at least 10mm wide in case of recurrent disease or high-grade tumors (1). The clinical course and the history of this patient clearly demonstrate that achieving free resection margins at the first surgery is of paramount importance for definitive tumor eradication. Indeed, the following procedures never achieved free resection margins and allowed the tumor to reach deeper bone infiltration and to worse the histological grading, in fact the first diagnosis was keratoacanthoma, but the subsequent histological examination demonstrated the presence of cSCC; moreover, repetitive surgery and radiotherapy were probably the cause for infection overlap. A multidisciplinary approach involving the thoracic surgeon was in the end necessary to perform extensive chest wall resection involving the lower sternum, and multiple costal arches and the upper part of the abdominal wall. The correct reconstruction technique became the most critical part of the procedure to avoid post-operative complications such as respiratory failure with chest wall instability, wound infection and herniation of thoracic and abdominal organs (7,8). Moreover, pectoralis muscle flaps were not suitable because they were previously compromised, but the previous left VRAM was in good condition and free from neoplastic involvement, thus we decide to reuse it for final reconstruction. The chronic infection of the surgical site made the situation even more complex. We then decided to use a combination of different biological and synthetic materials. The omentum was prepared to cover the reconstruction of the thoracic wall and diaphragm, performed with Gore-Tex® Dualmesh®, to reduce the risk of infection. The omentum is very effective in improving lymphatic drainage and blood supply of infected surgical wounds (8,9). Moreover, the omentum was used to guarantee the vascular support to the following reconstructions performed by using the previous VRAM, and bilateral latissimi dorsi myocutaneous flaps. In this case, we decided to limit the usage of synthetic material to the Dualmesh® prosthesis and to use extensively well vascularized myocutaneous flaps, due to the presence of infection and necrotic tissues. Arnold et al demonstrated that most patients can tolerate sternectomy or resection of 4–6 ribs at the cartilage level without experiencing flail chest or respiratory insufficiency (10). The best technique and material for chest wall resection is still not defined (7,8,10). In this case the use of biological tissues is very effective to reduce the risk of post-operative infection and thus failure of the reconstruction; moreover, the combination of well tight Gore-Tex® Dualmesh® with latissimus dorsi flap guarantee an optimal respiratory support in case of lower resection of the sternum and anterior costal arches (11-13).
Conclusions
Cutaneous squamous cell carcinoma of the chest is a very rare disease that can recur if resected with insufficient resection margins. At each recurrence of the disease, the tumor can reach ever deeper anatomical levels and when sternum or ribs became infiltrated very extensive resection is required. Different techniques and material are available for such complex reconstruction. A multidisciplinary team is very important to achieve optimal results.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Stratigos A, Garbe C, Lebbe C, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guidelines. Eur J Cancer 2015;51:1989-2007. [Crossref] [PubMed]
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: A review of high-risk and metastatic disease. Am J Clin Dermatol 2016;17:491-508. [Crossref] [PubMed]
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep 2017;11:136. [Crossref] [PubMed]
- Schmults CD, Karia PS, Carter JB, et al. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol 2013;149:541-7. [Crossref] [PubMed]
- Brantsch KD, Meisner C, Schonfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 2008;9:713-20. [Crossref] [PubMed]
- Ferrigno P, Monaci N, Pangoni A, et al. The video shows all the steps of the operation that are described in the text. Asvide 2020;7:023. Available online: http://www.asvide.com/watch/33070
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Sanna S, Brandolini J, Pardolesi A, et al. Materials and techniques in chest wall reconstruction: a review. J Vis Surg 2017;3:95. [Crossref] [PubMed]
- Mazzaferro D, Song P, Massand S, et al. The omental free flap-A review of usage and physiology. J Reconstr Microsurg 2018;34:151-69. [Crossref] [PubMed]
- Arnold PG, Pairolero PC. Chest wall reconstructions: an account of 500 consecutive cases. Plast Reconstr Surg 1996;98:804-10. [Crossref] [PubMed]
- Hameed A, Akhtar S, Naqvi A, et al. Reconstruction of complex chest wall defects by using polypropylene mesh and a pedicled latissimus dorsi flap: a 6-year experience. J Plast Reconstr Aesthet Surg 2008;61:628-35. [Crossref] [PubMed]
- Merritt RE. Chest wall reconstruction without prosthetic material. Thorac Surg Clin 2017;27:165-9. [Crossref] [PubMed]
- Losken A, Thourani VH, Carlson GW, et al. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg 2004;57:295-302. [Crossref] [PubMed]