Tube thoracostomy; chest tube implantation and follow up
Introduction
Tube thoracostomy is the most commonly performed surgical procedure in thoracic surgery.
It is defined as insertion of a tube (chest tube) into the pleural cavity to drain air, blood, bile, pus, chyle or other fluids (1).
The first description of thoracostomy begins with Hippocrates (2). In the 14th century, some surgeons, such as Guy de Chauliac, used it for the management of chest trauma, without anesthesia. In the early 18th centery, Boerhaave recognized the need to evacuate the hemothorax that resulted from penetrating wound of the chest (3), and in 1876, Hewett (4) was the first to use a completely closed intercostal drainage system, but it was not until World War II that tube thoracostomy became common in the treatment of injured patients (5).
Contraindications
Absolute contraindications: Published guidelines state there are no absolute contraindications for drainage via tube thoracostomy, except when a lung is completely adherent to the chest wall throughout the hemithorax, or upon patient refusal (6).
Relative contraindications: risk of bleeding due to coagulopathies or anticoagulation medications, and infection overlying the insertion site. Whenever possible, coagulopathies and platelet defects should be corrected with the infusion of blood products prior to the procedure. Overlying cellulites or herpes zoster infections should be avoided by choosing another puncture site. Other relative contraindications include multiple pleural adhesions, emphysematous blebs and scaring.
The procedure should be always explained fully to the patient before taking written consent. Written consent should be always obtained, except in emergency situations.
Chest drain insertion has been reported to be a painful procedure with 50% of patients experiencing pain level of 9-10 on a scale of 10, and therefore premedication, such as local anesthetic (1-2% Lidocaine), should be given.
As a chest drain may remain in situ for several days, aseptic technique is essential to avoid wound site infection or secondary empyema.
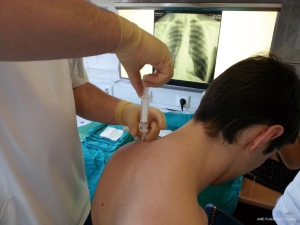
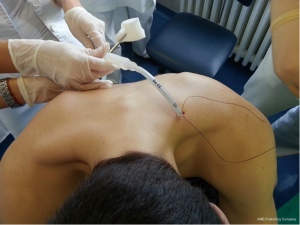
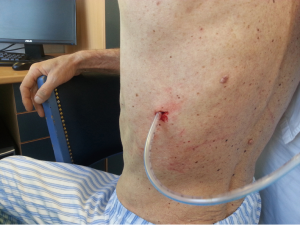
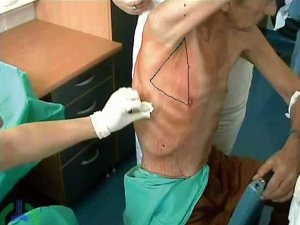
British Thoracic Society (BTS) has recommended the “Triange of safety” (Figure 1) as the site for insertion for intercostal drain (7). This area is bordered by the anterior border of the latissimus dorsi, the lateral border of the pectoralis major muscle, a line superior to the horizontal level of the nipple, and an apex below the axilla. Most surgeons insert the chest tube via an incision at the 4th or 5th intercostals space in the anterior axillary or mid-axillary line, as the innermost layer of intercostals muscle being poorly developed at this point, and comprising thin intercostals, which blend with the internal intercostals layer except where separated by neurovascular bundles. To avoid neurovascular bundle, it is normally advocated that the drain be located in the interspace just too superior margin to the lower rib (8).
The patient should be positioned supine or at 45° angle (elevation the patient lessens the risk of diaphragm elevation and consequent misplacement of the chest tube into the abdominal space. The arm on the affected side should be abducted and externally rotated, simulating a position in which the palm of the hand is behind the patient’s head, which will spread the intercostals space.
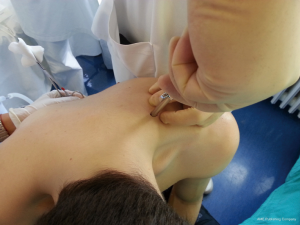
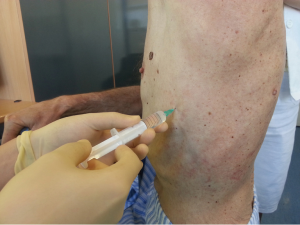
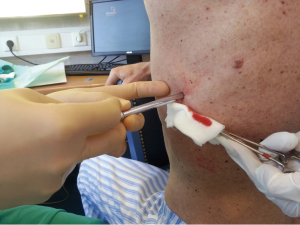
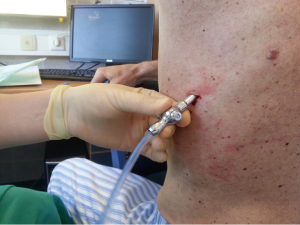
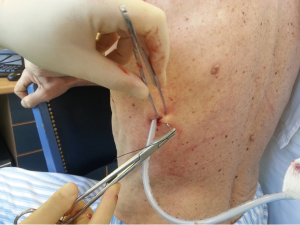
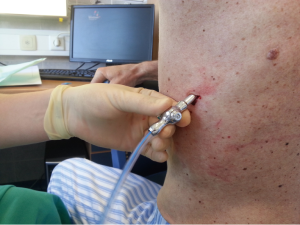
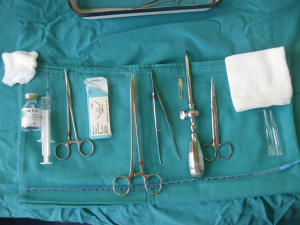
After sterilization of the area with betadine solution, local anaesthetic (5 mL of 1% lidocaine solution) should be infiltrated to the skin with 25-gauge needle. Using continued negative suction as the needle advances, confirmation of entery into the pleural space will be filling the syringe with air. An incision of about 2 cm is made parallel to the upper margin of lower rib, following blunt dissection with dissection clamp, the pleural space is penetrated. Any parenchymal adhesions, if present, can be eliminated through finger exploration.
There are three techniques most commonly used to place a chest tube. The standard technique employs blunt dissection to access the pleural space. The Seldinger technique uses serial dilatation over a guide wire and third technique is by using trocar.
When using the trocar, significant force should never be used, as this risks sudden chest penetration and damage to essential intra-thoracic structures. The trocar technique is associated with a higher rate of intrathoracic organ injury (9).
The Advanced Trauma Life Support course sponsored by the American College of Surgeons recommends the clamp and finger technique. Trocar insertion or pigtail catheter insertion using the Seldinger technique is reserved for experienced surgeons familiar with chest wall anatomy
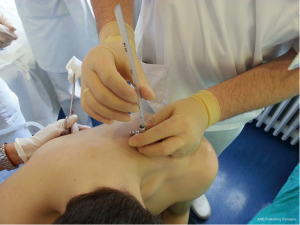
In Seldinger technique, after insertion of an introducer needle into the pleural space, a guide wire is inserted through the introducer needle, and after using dilators a chest tube is inserted into the pleural space.
In trocar technique, after penetrating into pleural space, the trocar and drain together are advanced towards the apex by using force.
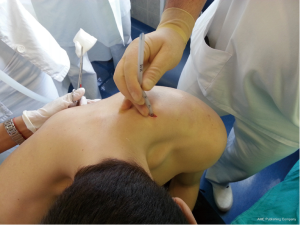
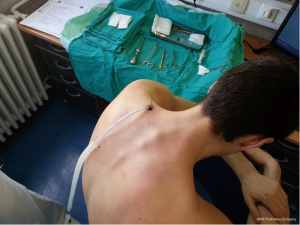
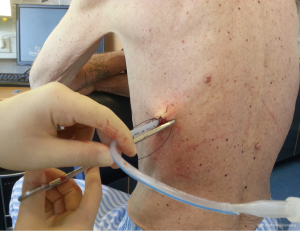
Chest drains should be secured with 1.0 silk suture anchored to the skin and the drain with a suitable non slip knot technique.
Some surgeons avoid insertion of chest tube at the second intercostal space in the mid-clavicular line, as this requires dissection through the pectoralis muscle and glandular tissue in female patient, which can lead to the serious infection (mastitis), leaves a visible scar, and often don’t have fully control of pleural space.
For evacuation of an apical pneumothorax, an alternative site can be apical access through the first intercostals space in scapular line. During the procedure it is important not to access trocar anteriorly, due to large subclavian vessels can be seriously injured. This access is quite comfortable for the patient and easy to handle. Usually it requires experienced thoracic surgeons to perform.
The operator can assume that the drain is in the pleural cavity if:
- The chest drain fogs up;
- The fluid level in the chest drain internal pipe swing;
- The prime level fluid bubbles when patient coughs;
- Pleural fluid drains from the chest.
Recent literature suggests that treatment with small caliber tube thoracostomy is equally effective and less painful then treatment with large caliber tubes. There are several varieties of tubes for thoracostomy. Small pigtail catheters (10-14 F) placed with wire guidance have become increasingly popular. Studies comparing wire-guided technique with small-bore (<14 F) and large-bore (>14 F) catheters have demonstrated that small bore catheters are more accurate (10) with no disadvantage in clinical outcome (11,12).
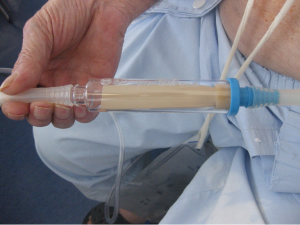
Once the chest tube has been inserted, it must be connected to either suction or an apparatus to allow unidirectional drainage (water seal without suction or a Heimlich valve). This is usually the closed underwater seal bottle in which a tube is placed under water, which enables the operator to see air bubbles, but it include obligatory inpatient management, difficulty of patient mobilization and the risk of knocking over the bottle.
Recent randomized trials following pulmonary resection, when air leak is common, showed that applying suction to chest tubes should be avoided. Most patients with small to moderate size air leks benefit from early conversion (first 24 hours) to water seal (13-15). There is no evidence to support the routine initial use of suction in the treatment of spontaneous pneumothorax or in sealing air leaks or reducing hospital stay.
One popular method is to use a commercially available three-chamber water seal system (16). The three-chamber device drains the chest tube to a collection chamber that is sealed by middle water chamber. The water seal chamber contains a gauge that demonstrates the degree of air leak in the system. Wall suction tubing is connected to the third (suction) chamber. A regulator built into the device controls the vacuum in the suction chamber. A low-level vacuum (5-20 cm H2O) is recommended in most circumstances. A stronger vacuum will increase the flow through the system in a diminishing manner (17).
A simple one-way valve (Heimlich valve) may be attached to the chest tube to allow the patient freedom from wall suction. However, this will accomplish only passive, suctionless drainage.
Tube thoracostomy is an invasive procedure and complications can result due to inadequate knowledge of thoracic anatomy or inadequate training and experience.
Complications of tube thoracostomy can be classified as either technical or infective. Technical causes include tube malposition, blocked drain, chest drain dislodgement, reexpansion pulmonary oedema, subcutaneous amphysema, nerve injuries, cardiac and vascular injuries, oesophageal injuries, residual/postextubation pneumothorax, fistulae, tumor recurrence at insertion site, herniation through the site, chylothorax and cardiac dysrhythmias. Infective complications include empyema and surgical site infections including cellulites and necrotizing fasciitis.
A survey of junior residents on the anatomical landmarks when inserting an intercostals drain revealed that 45% were placed outside the safe area of chest drain insertion with the most common error (20%) being a choice of insertion too low (18). When a chest drain is placed too low, there is a high probability of abdominal placement, and chest tube will not only perforate the diaphragm but also will damage intra-abdominal organs.
A 2006 meta-analysis confirmed that 24-hour regimen of a first-generation cephalosporin significantly reduces postinsertion pneumonia and empyema in trauma patients (19). However, the use of prophylactic antibiotics has been found unnecessary in patients with primary spontaneous pneumothorax (20).
Silastic tubes are preferred because older tubes have fewer drainage holes, are not well visualized on chest radiographs and produce more pleural inflammation. Silastic chest tubes contain a radiopaque strip with gap that serves to mark the most proximal drainage hole.
There is no evidence to suggest that clamping a chest drain prior to its removal increases success or prevent recurrence of a pneumothorax and may be hazardous. By clamping the chest drain for several hours (4-6 hours), followed by a chest radiograph, a minor air leak may be detected, avoiding the need for later chest drain reinsertion.
Prior to removal of a chest tube, the chest radiograph must show complete expansion of the lung and there should be no air leaks during coughing or suction. Chest tube should be removed with the patient performing a valsalva maneuver or during expiration. A repeat chest radiograph can rule out a pneumothorax, which can occasionally occur during removal.
The rate of complications associated with tube thoracostomy is from 3% to 18% (21). In patients with pneumothorax, placement of a chest tube should be safer, since there is a space between the chest wall and the lung. Complication rates of tube thoracostomy have been found to be higher in critically ill patients with about 21% of tube placed intrafissurally and 9% intraparenchymally (22). Trocar technique of chest tube insertion has been shown to increase the risk of complications such as tube malposition, compared with the blunt dissection technique (23).
Chest tube malposition is the most common complication and has been defined by CT confirmation in four locations: intraparenchymal, fissural, extrathoracic and angulation of the drain in the pleural space (24).
- Intraparenchymal tube placement occurs more likely in the presence of pleural adhesions or preexisting pulmonary disease. It can be dramatic if there is associated injury to pulmonary vessels. However, clinical manifestation may be absent and the only clue to the diagnosis may be inadequate drainage of air and fluid.
- Fissural (interlobar) tube placement is significantly higher when using the lateral approach of tube thoracostomy, comparing to the anterior approach. Malfunctioning interfissural tubes should be repositioned or replaced to improve function.
- Chest wall (subcutaneous) tube placement is a rare complication with reported incidence between 1-1.8% (25). An unstable chest wall secondary to multiple rib fractures, haemathoma and hurried chest tube insertion are the most common factors (26). It can be identified clinically by the lack of fluctuation of the fluid level in the drainage system, and radiologically by subcutaneous position of the chest tube. Subcutaneous tube should be removed and replaced correctly into the pleural cavity.
- Mediastinal tube placement can result in perforation of heart, injuries to large vessles, perforation of the esophagus and nerve injuries. Perforation of the heart is a rare catastrophic complication and injuries of both atrii and ventricles have been reported (27-30). Predisponing factors in patients reported were thoracic deformities, enlarged cardiac chambers, trauma, and postpneumonectomy space. Injury to the pulmonary artery is a rare but serious complication of chest tube insertion (31). This injury occurred mainly with the use of trocar method, compared with dense pleural adhesions in pleural space or postpneumonectomy space. Injury to the subclavian artery may occurred using apical access through the first intercostals space in scapular line with trocar. Nerve injuries (Horner syndrome, phrenic nerve, ulnar nerve) are rare and mostly reported in paediatric population.
Development of subcutaneous emphysema is a known complication of tube thoracostomy. It is usually presented as subcutaneous crepitation demonstrable clinically or as an occult radiological finding. Extensive subcutaneous emphysema may present with extreme discomfort, disfigurement, anxiety, upper airway obstruction.
Intercostal artery can be injuried during the insertion of chest tube. Intercostal arteries may bleed profusely when traumatized. Dissection during tube insertion should be done above the superior border of the rib to avoid the neurovascular bundles on the groove located on the inferior aspect. If traumatized, thoracotomy for haemostasis is required.
Abdominal placement of chest tube usually occurs when tube thoracostomy is performed too low, below the “Triangle of safety”. Injuries to the spleen, liver and stomach have been reported, as well as in cases of acquired diaphragmatic rupture with visceral herniation (32). Injury to hollow viscus requires surgical repair. The extent of surgery on a perforated viscus depends on the degree of injury.
Blocked chest tube (nonfunctional) may be due to kinking, angulation, clot formation within the lumen or the presence of debris. A cardinal sign of blocked chest tube is failure of fluid within the tube to fluctuate with coughing or respiration. Tension pneumothorax can also result in cases of ongoing air leak.
Chest tube dislodgement can be partial or total. It can be prevented by meticulous care and good technique of drain anchorage. The ideal suture to secure the chest tube should be strong and nonabsorbable (1.0 silk).
Reexpansion pulmonary edema is an uncommon but fatal complication that can occur following tube thoracostomy, with mortality rate up to 20% (33). The most important pathophysiological mechanism appers to be increased endothelial permeability and loss of integrity of the alveolar capillaries, leading to exudation of protein-rich fluid. The risk factors for developing edema in patient include young age (<40 years), collapse of the affected lung for more than three days, large pneumothorax (>30% of single lung), application of significant negative pressure suction and rapid lung reexpansion (34). Patient usually becomes symptomatic within 2 hours after rapid reexpansion. There may be frothy sputum production associated with tachypnea, tachycardia and cyanosis. The goal should be to keep the pleural pressure above –20 cm H2O.
Residual/postextubation pneumothorax can be avoided during the removal of chest tube by maintaining a sustained valsalva manoeuvre to forcibly inflate the lung against the chest wall with breathing suspended until the purse string is tied. Repeat tube thoracostomy is indicated if pneumothorax is significant or if it is secondary to persistent air leak. Small residual pneumothorax requires no intervention.
Infectious complication-closed tube thoracostomy is classified s clean contaminated and hence risk of infection of wound is 7.7%. Studies of empyema secondary to tube thoracostomy have reported complication rates from 1% to 25% (35). Empyema occurs more frequently after penetrating chest trauma than blunt chest trauma as penetrating injuries allow direct entry of microorganisms into the pleural space (36-50). Necrotizing fasciitis after tube thoracostomy has been reported complicating secondary spontaneous pneumothorax for tuberculosis (48,51-64) (Figures 1-20).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986;17:309-12. [PubMed]
- Hippocrates. Writing. In: Hutchins RM. eds. Great books of the western world. Vol 10. Chocago: Encyclopedia Brittanica;1952:142.
- Monaghan SF, Swan KG. Tube thoracostomy: the struggle to the “standard of care”. Ann Thorac Surg 2008;86:2019-22. [PubMed]
- Hewett FC. Thoracentesis: The Plan of Continuous Aspiration. Br Med J 1876;1:317. [PubMed]
- Ball CG, Lord J, Laupland KB, et al. Chest tube complications: how well are we training our residents? Can J Surg 2007;50:450-8. [PubMed]
- Dev SP, Nascimiento B Jr, Simone C, et al. Videos in clinical medicine. Chest-tube insertion. N Engl J Med 2007;357:e15. [PubMed]
- Laws D, Neville E, Duffy J, et al. BTS guidelines for the insertion of a chest drain. Thorax 2003;58 Suppl 2:ii53-9. [PubMed]
- Ellis H. The applied anatomy of chest drain insertion. Br J Hosp Med (Lond) 2007;68:M44-5. [PubMed]
- Dural K, Gulbahar G, Kocer B, et al. A novel and safe technique in closed tube thoracostomy. J Cardiothorac Surg 2010;5:21. [PubMed]
- Protic A, Barkovic I, Bralic M, et al. Targeted wire-guided chest tube placement: a cadaver study. Eur J Emerg Med 2010;17:146-9. [PubMed]
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010;137:536-43. [PubMed]
- Rivera L, O’Reilly EB, Sise MJ, et al. Small catheter tube thoracostomy: effective in managing chest trauma in stable patients. J Trauma 2009;66:393-9. [PubMed]
- Cerfolio RJ. Recent advances in the treatment of air leaks. Curr Opin Pulm Med 2005;11:319-23. [PubMed]
- Cerfolio RJ, Bryant AS, Singh S, et al. The management of chest tubes in patients with a pneumothorax and an air leak after pulmonary resection. Chest 2005;128:816-20. [PubMed]
- Reed MF, Lyons JM, Luchette FA, et al. Preliminary report of a prospective, randomized trial of underwater seal for spontaneous and iatrogenic pneumothorax. J Am Coll Surg 2007;204:84-90. [PubMed]
- Durai R, Hoque H, Davies TW. Managing a chest tube and drainage system. AORN J 2010;91:275-80. [PubMed]
- Baumann MH. What size chest tube? What drainage system is ideal? And other chest tube management questions. Curr Opin Pulm Med 2003;9:276-81. [PubMed]
- Griffiths JR, Roberts N. Do junior doctors know where to insert chest drains safely? Postgrad Med J 2005;81:456-8. [PubMed]
- Sanabria A, Valdivieso E, Gomez G, et al. Prophylactic antibiotics in chest trauma: a meta-analysis of high-quality studies. World J Surg 2006;30:1843-7. [PubMed]
- Olgac G, Aydogmus U, Mulazimoglu L, et al. Antibiotics are not needed during tube thoracostomy for spontaneous pneumothorax: an observational case study. J Cardiothorac Surg 2006;1:43. [PubMed]
- Chan L, Reilly KM, Henderson C, et al. Complication rates of tube thoracostomy. Am J Emerg Med 1997;15:368-70. [PubMed]
- Remérand F, Luce V, Badachi Y, et al. Incidence of chest tube malposition in the critically ill: a prospective computed tomography study. Anesthesiology 2007;106:1112-9. [PubMed]
- Huber-Wagner S, Körner M, Ehrt A, et al. Emergency chest tube placement in trauma care - which approach is preferable? Resuscitation 2007;72:226-33. [PubMed]
- Dural K, Gulbahar G, Kocer B, et al. A novel and safe technique in closed tube thoracostomy. J Cardiothorac Surg 2010;5:21. [PubMed]
- Bergaminelli C, De Angelis P, Gauthier P, et al. Thoracic drainage in trauma emergencies. Minerva Chir 1999;54:697-702. [PubMed]
- Ozpolat B, Yazkan R. Ectopic chest tube insertion to thoracic wall. Turk J of Geriatrics 2007;10:40-2.
- Kerger H, Blaettner T, Froehlich C, et al. Perforation of the left atrium by a chest tube in a patient with cardiomegaly: management of a rare, but life-threatening complication. Resuscitation 2007;74:178-82. [PubMed]
- Meisel S, Ram Z, Priel I, et al. Another complication of thoracostomy--perforation of the right atrium. Chest 1990;98:772-3. [PubMed]
- Kopec SE, Conlan AA, Irwin RS. Perforation of the right ventricle: a complication of blind placement of a chest tube into the postpneumonectomy space. Chest 1998;114:1213-5. [PubMed]
- Abad C, Padrón A. Accidental perforation of the left ventricle with a chest draintube. Tex Heart Inst J 2002;29:143. [PubMed]
- Takanami I. Pulmonary artery perforation by a tube thoracostomy. Interact Cardiovasc Thorac Surg 2005;4:473-4. [PubMed]
- Içöz G, Kara E, Ilkgül O, et al. Perforation of the stomach due to chest tube complication in a patient with iatrogenic diaphragmatic rupture. Acta Chir Belg 2003;103:423-4. [PubMed]
- Mahfood S, Hix WR, Aaron BL, et al. Reexpansion pulmonary edema. Ann Thorac Surg 1988;45:340-5. [PubMed]
- Matsuura Y, Nomimura T, Murakami H, et al. Clinical analysis of reexpansion pulmonary edema. Chest 1991;100:1562-6. [PubMed]
- Chan L, Reilly KM, Henderson C, et al. Complication rates of tube thoracostomy. Am J Emerg Med 1997;15:368-70. [PubMed]
- Hsu SP, Wang HC, Huang IT, et al. Tube thoracostomyrelated necrotizing fasciitis: a case report. Kaohsiung J Med Sci 2006;22:636-40. [PubMed]
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6 Suppl 1:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6 Suppl 1:S7-20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6 Suppl 1:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6 Suppl 1:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6 Suppl 1:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6 Suppl 1:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6 Suppl 1:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6 Suppl 1:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6 Suppl 1:S162-6. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6 Suppl 1:S152-61. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6 Suppl 1:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4 Suppl 1:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]
- Papaioannou M, Pitsiou G, Manika K, et al. COPD Assessment Test: A Simple Tool to Evaluate Disease Severity and Response to Treatment. COPD 2014;11:489-95. [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-107. [PubMed]
- Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014;6 Suppl 1:S146-51. [PubMed]
- Zarogoulidis P, Porpodis K, Kioumis I, et al. Experimentation with inhaled bronchodilators and corticosteroids. Int J Pharm 2014;461:411-8. [PubMed]
- Bai C, Huang H, Yao X, et al. Application of flexible bronchoscopy in inhalation lung injury. Diagn Pathol 2013;8:174. [PubMed]
- Zarogoulidis P, Kioumis I, Porpodis K, et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 2013;7:1115-34. [PubMed]
- Zarogoulidis P, Pataka A, Terzi E, et al. Intensive care unit and lung cancer: when should we intubate? J Thorac Dis 2013;5 Suppl 4:S407-12. [PubMed]
- Hohenforst-Schmidt W, Petermann A, Visouli A, et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug Des Devel Ther 2013;7:627-33. [PubMed]
- Zarogoulidis P, Kontakiotis T, Tsakiridis K, et al. Difficult airway and difficult intubation in postintubation tracheal stenosis: a case report and literature review. Ther Clin Risk Manag 2012;8:279-86. [PubMed]
- Zarogoulidis P, Tsakiridis K, Kioumis I, et al. Cardiothoracic diseases: basic treatment. J Thorac Dis 2014;6 Suppl 1:S1. [PubMed]
- Kolettas A, Grosomanidis V, Kolettas V, et al. Influence of apnoeic oxygenation in respiratory and circulatory system under general anaesthesia. J Thorac Dis 2014;6 Suppl 1:S116-45. [PubMed]
- Turner JF, Quan W, Zarogoulidis P, et al. A case of pulmonary infiltrates in a patient with colon carcinoma. Case Rep Oncol 2014;7:39-42. [PubMed]
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol 2013;8:194. [PubMed]
- Tsakiridis K, Zarogoulidis P. An interview between a pulmonologist and a thoracic surgeon-Pleuroscopy: the reappearance of an old definition. J Thorac Dis 2013;5 Suppl 4:S449-51. [PubMed]
- Huang H, Li C, Zarogoulidis P, et al. Endometriosis of the lung: report of a case and literature review. Eur J Med Res 2013;18:13. [PubMed]