
Recovery of pulmonary function after lung wedge resection
Introduction
Lung wedge resection is a non-anatomical resection that differs from a lobectomy or segmentectomy. Lung wedge resection is indicated for several diseases such as lung cancer, metastatic lung tumors, benign lung tumors, and non-definitive diagnosed nodules. Wedge resection in lung cancer is sometimes indicated in patients with marginal pulmonary function, high risk patients and elderly patients (1). If the phase III study of wedge resection in peripheral ground glass opacity dominant lung cancer demonstrates a positive result, it is expected that the indications for wedge resection will be expanded (2).
The prediction of postoperative pulmonary function is important for the evaluation for operative tolerance, the risk of postoperative complications, long-term quality of life of the patient, to determine the resectable volume of the lung and to plan the surgical procedure (3,4). The prediction of postoperative pulmonary function is calculated from the number of resected lung segments in cases of lobectomy or segmentectomy. Since wedge resection is non-anatomical, it is unclear as to what extent the lung volume can be practically resected, and there is no standard method to calculate the predicted postoperative pulmonary function. Some studies have reported that a lobectomy resulted in a greater decrease in postoperative pulmonary function than segmentectomy or wedge resection (5,6). However, the details of postoperative pulmonary function after lung wedge resection are not well understood. It is therefore necessary to understand how the patient’s pulmonary function is altered with time and determine the extent of recovery.
The aim of this study was to assess the influence of wedge resection on postoperative pulmonary function.
Methods
Patients
Wedge resection was indicated for patients with suspicious benign nodules, metastatic lung tumors, an inflammatory lung nodule and pre-invasive adenocarcinoma of the lung that was diagnosed using radiological findings in case of pure ground glass nodule or part solid ground glass nodule with the solid component of less than 5 mm. Patients who had undergone any lung resection were asked to attend postoperative pulmonary function tests (PFTs) at 3, 6 and 12. Patients who had undergone lung wedge resection between January 2016 and December 2017 at the Jikei University Hospital in Tokyo, Japan, were retrospectively analyzed and those patients whose data included four PFTs (preoperative and postoperative 3, 6, and 12 months) were included in this study. Patients who missed any of the PFTs were excluded. Patient clinical demographic and perioperative information, including age, sex, smoking history, comorbidities, lung computed tomography (CT) findings, past history of thoracotomy, disease, size of the resected lesions, procedure (thoracotomy or thoracoscopic surgery, number and location of resections), operative time, intrathoracic adhesion, number, total length of staplers used, use of absorbability sheet and fibrin glue, postoperative complications, preoperative and postoperative PFTs [vital capacity (VC) and forced expiratory volume in one second (FEV1)] and details of the leading surgeon were collected from a prospective database or the patients’ medical records. The %VC and %FEV1 (measured value/standard value rate) were calculated with the LMS method, which was modified for Japanese patients, as recommended by the Japanese Respiratory Society (7).
Ethical considerations
Informed consent was obtained from all patients after explaining the surgical indication, risks, and benefits, and other surgical procedures and treatments. All procedures that were performed during this study were in accordance with the ethical standards of our institutional review board and the Declaration of Helsinki and its later amendments. The Ethics Committee of Jikei University School of Medicine approved this study (approval number: 30-243 [9264]) and waived the need for obtaining individual patient consent for this retrospective study.
Statistical analyses
We calculated the differences among the preoperative value and the postoperative values of VC and FEV1 at each time point (3, 6, and 12 months), which are expressed as ΔVC and ΔFEV1, respectively. We also calculated the postoperative/preoperative pulmonary function ratios of the VC and FEV1 values at each time point, which are expressed as recovery ratios (RRs), in order to evaluate the chronological change in the ratio from baseline in each patient. We compared the values of the VC or FEV1 and the RRs of the VC or FEV1 among the time points using a paired t-test. Paired t-tests for the RRs of the VC or FEV1 were performed by setting the preoperative value to 100%. A 120 mL decrease in VC and FEV1 was considered to be clinically meaningful when comparing different of preoperative and postoperative value. We calculated that a sample size of 24 patients would provide a power of 80% in the detection of a 120 mL mean decrease in the VC and FEV1, with a type I error of 5%. For power analysis, we used a standard deviation (SD) of 200 mL of ΔVC and ΔFEV1. We assessed the correlations between clinical factors and the RRs of the VC or FEV1 at 12 months postoperatively using an independent t-test in order to elucidate risk factors for loss of pulmonary function. We used JMP version 13.0.0 (SAS Institute, Cary, NC, USA) to perform the statistical analyses. P values <0.05 were considered statistically significant.
Results
Patient characteristics
One-hundred and twenty patients underwent lung wedge resection and of those, 29 patients had available and complete PFTs data. Reasons for excluded cases were that patients did not agree to undergo regular PFTs, patients did not visit for follow-up, follow-up was completed ahead of the 12 month time frame or the doctor in charge omitted tests at his/her discretion.
Table 1 provides the characteristics of the 29 patients. The mean and SD of the age at the time of surgery was 66±15 years. Fifteen patients had respiratory comorbidity and of those five patients had more than two comorbidities. The means and SDs of the preoperative %VC and %FEV1 were 92.1%±13.4% and 87.3%±15.4%, respectively. Twelve patients had lung cancer and the reasons of indication for wedge resection were suspicious pre-invasive lesion in 7 patients and borderline pulmonary function or other high risk factors in 5 patients. The mean and SD of the size of the resected lesions (the size of the largest lesion was taken in cases of multiple lesions) was 16±7 mm. All patients underwent thoracoscopic surgery, but intraoperative conversion to thoracotomy was necessary in one patient due to severe intrathoracic adhesion. Absorbable reinforcement sheets were used in 14 patients, and fibrin glue was also used in eight of these patients. Although one patient experienced postoperative pleuritis, he underwent chest tube thoracostomy and improved. The remaining patients experienced no postoperative complications. Our surgical team included staff surgeons and no residents. In this study, seven surgeons conducted surgeries as operators.
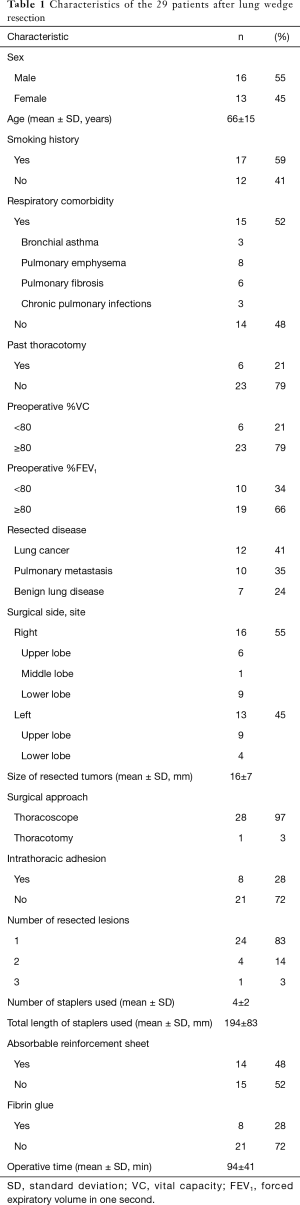
Full table
If these 29 patients had not undergone surgery, the mean reductions in the VC and FEV1 of standard value, as calculated with the LMS method, over the course of 12 months were 22±9 and 20±7 mL, respectively.
Recovery of the VC
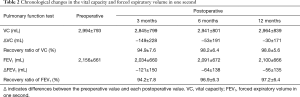
The means and SDs of the preoperative and 3-, 6-, and 12-month postoperative VC values were 2,994±793, 2,845±799, 2,941±801, and 2,964±839 mL, respectively (Table 2, Figure 1A). The ΔVC values at 3, 6, and 12 months postoperatively were −149±228, −53±191, and −30±171 mL, respectively (Table 2, Figure 1B). The RRs of the VC at 3, 6, and 12 months postoperatively were 94.9%±7.6%, 98.2%±6.4%, and 98.8%±5.6%, respectively (Table 2, Figure 1C). The VC at 3 months postoperatively was significantly reduced compared to the preoperative VC (P=0.002). The VC had recovered significantly at 6 and 12 months postoperatively (P=0.003 and 0.003, respectively). The RR of the VC at 3 months postoperatively was significantly lower compared to the baseline (P=0.001). The RR of the VC increased gradually at 6 and 12 months postoperatively, and reached a point where they did not significantly differ compared to the baseline (P=0.150 and 0.254, respectively).
Full table

Recovery of the FEV1
The means and SDs of the preoperative and 3-, 6-, and 12-month postoperative FEV1 were 2,156±661, 2,034±660, 2,091±672, and 2,100±666 mL, respectively (Table 2, Figure 2A). The ΔFEV1 values at 3, 6, and 12 months postoperatively were −121±150, −64±138, and −56±135 mL, respectively (Table 2, Figure 2B). The RRs of the FEV1 at 3, 6, and 12 months postoperatively were 94.2%±7.8%, 96.9%±6.3%, and 97.2%±6.4%, respectively (Table 2, Figure 2C). The FEV1 was significantly reduced at 3 months postoperatively compared to the preoperative value (P<0.001). The FEV1 had recovered significantly at 6 and 12 months postoperatively (P=0.015 and 0.009, respectively). However, the FEV1 at 6 and 12 months postoperatively remained significantly lower than the preoperative value (P=0.020 and 0.036, respectively). The RR of the FEV1 at 3 months postoperatively was significantly lower compared to the baseline (P=0.001). Although the RR of the FEV1 increased gradually, the RRs at 12 months remained significantly lower compared to the baseline (P=0.014 and 0.029, respectively).

Factors related to postoperative pulmonary function
Table 3 presents the results of univariate analysis of the factors related to changes in the pulmonary function at 12 months postoperatively compared to the preoperative value. The RR of the VC was significantly preserved in patients with tumors ≥20 mm in size compared to that in patients with tumors <20 mm in size (101.7% vs. 97.0%, respectively; P=0.027). Although the difference was not significant, the VC was relatively preserved in patients aged <70 years compared to that in patients aged ≥70 years (100.0% vs. 96.7%, respectively; P=0.079). The RR of the FEV1 was significantly preserved in patients aged <70 years compared to that in patients aged ≥70 years (99.5% vs. 94.5%, respectively; P=0.035). Preoperative %VC and %FEV1 were not significant factors for the recovery rate of VC and FEV1 from the baseline.
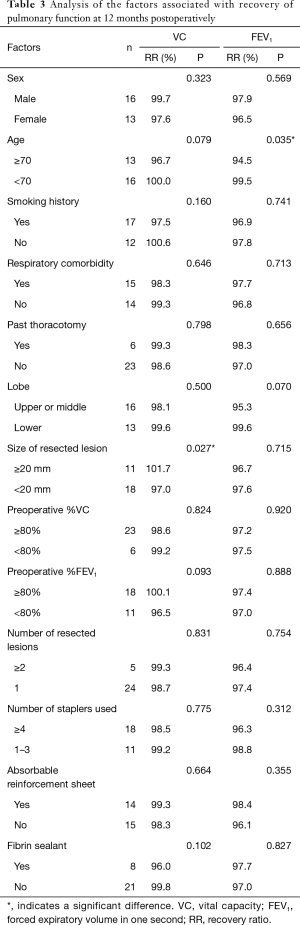
Full table
Discussion
The aim of this study was to evaluate the influence of wedge resection on postoperative pulmonary function, which has not been fully elucidated previously. There were two major findings in this study. First, the VC after lung wedge resection decreased temporarily but recovered to near the preoperative level after 12 months. Secondly, the FEV1 after lung wedge resection decreased and recovered gradually over the course of 12 months but did not recover to preoperative level.
In this study, the VC after lung wedge resection decreased temporally and the RR of the VC at 3 months postoperatively was approximately 5%. The VC then recovered to near the preoperative level (approximately 99%) after 12 months. The mean loss of VC of standard value, as calculated with the LMS method, over the course of 12 months was 22±9 mL without lung resection, and the actual postoperative VC loss was −30±171 mL at 12 months. These results led us to believe that the potential volume of the remaining lung can compensate for the volume of the resected lung, to some extent. A previous study also reported the compensatory response of the remaining lung after lung resection and supports our conclusion of these results (8). In cases wherein the resected lung volume is small amount, such as in wedge resection compared to lobectomy, the VC will recover almost completely due to compensation. It can be concluded that the loss of VC after lung wedge resection is minimal.
The FEV1 following lung wedge resection decreased at 3 months postoperatively and recovered gradually from 6–12 months. However, the FEV1 did not recover to the preoperative level, in contrast to the VC. The mean loss of FEV1 of standard value, as calculated with the LMS method, over the course of 12 months was 20±7 mL without lung resection, and the actual postoperative FEV1 loss at 12 months was −56±135 mL, approximately twice the standard calculated decrease. We hypothesized that the difference in the degree of recovery of VC and FEV1 suggests that damage to the respiratory muscles through surgery affects the FEV1 more than the VC. Another study has compared postoperative PFTs between wedge resection and mediastinal procedure without lung resection and demonstrated that there was no significant difference in decrease of the PFTs index (9). A previous study reported that in patients with low FEV1, a more invasive approach increased the postoperative complication rate (10). Although the relation is indirect, we hypothesized that damage to the respiratory muscle is a factor associated with a decrease in FEV1.
The clinical factor of younger age was related to greater postoperative recovery of pulmonary function, in relation to both the VC and the FEV1. We surmised that the potential volume of the remaining lung to compensate for postoperative pulmonary function is more preserved in younger patients. This may be because such patients experienced fewer effects of decreasing static lung compliance due to the loss of elastic lung tissue, increasing chest wall stiffness, and decreasing respiratory muscle strength with age (11). Patients with larger tumors exhibited greater recovery of VC. We hypothesized that once the intrathoracic space that had been occupied by the lesion was resected, the remaining lung expanded into the space and began to function. However, this concept is paradoxical to the general idea that larger lesions would require larger resections, which would thus be associated with a poorer recovery.
These major findings clarified the influence of lung wedge resection on postoperative pulmonary function. These findings are beneficial for planning surgery and explaining the procedure to patients undergoing lung wedge resection.
This study had some limitations. First, of the 120 patients who underwent wedge resection, the data from four PFTs, preoperatively and at 3, 6, and 12 months postoperatively, were only available for 29 patients. Thus, this study was potentially affected by bias. Second, the 29 patients had relatively normal PFT and thus their recovery may not reflect the result of patients with worse preoperative values. Although it was demonstrated that preoperative PFT did not relate to recovery rate, further investigations would be appropriate. In addition, the follow-up period of only 12 months does not explore the long-term outcomes of this investigation. A previous study reported that patients undergoing lung resection, including lobectomy and sublobar resection, recovered up to 12 months postoperatively, but at >12 months postoperatively the absolute values decreased with age but the %VC or %FEV1 did not decrease (5). In a future study, we plan to investigate whether long-term follow-up of patients who underwent wedge resection will yield the same results. Finally, only the VC and FEV1 were used to assess postoperative pulmonary function in this study. Changes in the postoperative diffusing capacity or PFT in the immediate postoperative period, which have been reported to be useful predictors of postoperative risk, should be considered in future studies (12,13).
Conclusions
The VC following lung wedge resection decreased temporarily but recovered to near the preoperative level at 12 months postoperatively. The loss of VC after wedge resection is minimal. The FEV1 after lung wedge resection decreased and recovered gradually over the course of 12 months but did not recover to the preoperative level. These findings are beneficial for planning surgery and explaining the procedure to patients undergoing lung wedge resection.
Acknowledgments
The authors thank Editage for the English language review.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Jikei University School of Medicine (approval number: 30-243 [9264]), and the need for informed consent was waived.
References
- Alam NZ. Lung resection in patients with marginal pulmonary function. Thorac Surg Clin 2014;24:361-9. [Crossref] [PubMed]
- Suzuki K, Watanabe S, Wakabayashi M, et al. A nonrandomized confirmatory phase III study of sublobar surgical resection for peripheral ground glass opacity dominant lung cancer defined with thoracic thin-section computed tomography (JCOG0804/WJOG4507L). J Clin Oncol 2017;35:8561. [Crossref]
- British Thoracic Society. Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Colice GL, Shafazand S, Griffin JP, et al. American College of Chest Physicians. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- Kobayashi N, Kobayashi K, Kikuchi S, et al. Long-term pulmonary function after surgery for lung cancer. Interact Cardiovasc Thorac Surg 2017;24:727-32. [Crossref] [PubMed]
- Kim SJ, Lee YJ, Park JS, et al. Changes in pulmonary function in lung cancer patients after video-assisted thoracic surgery. Ann Thorac Surg 2015;99:210-7. [Crossref] [PubMed]
- Kubota M, Kobayashi H, Quanjer PH, et al. Clinical Pulmonary Functions Committee of the Japanese Respiratory Society. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig 2014;52:242-50. [Crossref] [PubMed]
- Ueda K, Hayashi M, Tanaka N, et al. Long-term pulmonary function after major lung resection. Gen Thorac Cardiovasc Surg 2014;62:24-30. [Crossref] [PubMed]
- Gu Z, Wang H, Mao T, et al. Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery. J Thorac Dis 2018;10:2331-7. [Crossref] [PubMed]
- Oparka J, Yan TD, Ryan E, et al. Does video-assisted thoracic surgery provide a safe alternative to conventional techniques in patients with limited pulmonary function who are otherwise suitable for lung resection? Interact Cardiovasc Thorac Surg 2013;17:159-62. [Crossref] [PubMed]
- Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J 1999;13:197-205. [Crossref] [PubMed]
- Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg 2008;85:1158-64. [Crossref] [PubMed]
- Varela G, Brunelli A, Rocco G, et al. Predicted versus observed FEV1 in the immediate postoperative period after pulmonary lobectomy. Eur J Cardiothorac Surg 2006;30:644-8. [Crossref] [PubMed]


