Impact of clinicopathological features on the efficacy of immune checkpoint inhibitors plus conventional treatment in patients with advanced lung cancer
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer death worldwide (1). In recent years, the advent of immunotherapy changed the landscape of treatment for lung cancer. Programmed-death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) are two most promising targets to release the brakes of T cells in effector phase and priming stage, respectively. Immune therapies targeting the programmed-death 1 (PD-1): PD-L1 axis achieved great success and durable anti-tumor response in non-small cell lung cancer (NSCLC). Promising results from Checkmate017, Checkmate057, Keynote010, OAK and POPLAR revolutionized the treatment paradigm of NSCLC in second or subsequent line (2-6). Keynote024 further pushed the pembrolizumab to the front-line treatment of patients with PD-L1 tumor proportion score (TPS) of more than 50% (7). In terms of small cell lung cancer (SCLC), the high frequency of somatic mutations prompts that the SCLC is a immunogenic type of cancer and possibly, responds to immunotherapy (8). Both nivolumab monotherapy and nivolumab plus ipilimumab showed promising anti-tumor activity with durable responses and manageable safety profiles in Checkmate032 (9). However, challenges have appeared. Only a small proportion of patients can respond to single-agent without a defined biomarker and PD-L1 is not a satisfied biomarker with several limitations so far. In this circumstance, to optimize the efficacy of immunotherapy and maximize the potential population that could benefit from immunotherapy, the idea of combining ICI with conventional therapies has been implemented and proved to be successful according to encouraging data from Keynote021 (10,11). More clinical trials were designed and recruited patients to testify the efficacy of various combinations including some of them were biomarker guided, for instance, PD-L1 expression and tumor mutation burden (TMB) (12).
Here, we performed a systematic review and meta-analysis to investigate the impact of clinicopathological features, including different type of ICI, PD-L1 expression and clinical characteristics on the efficacy of immune checkpoint inhibitors plus conventional treatment in patients with advanced lung cancer.
Methods
Search strategy
Up to March 2019, we performed a comprehensive electronic search on PubMed, EMBASE, Web of Science, Cochrane Library to screen the publications that reported the efficacy of combination therapy based on immune checkpoint inhibitors in lung cancer patients without any language restrictions. The search terms were limited to as followings: (“PD-1” OR “PD-L1” OR “CTLA-4” OR “immune checkpoint inhibitor” OR “nivolumab” “pembrolizumab” OR “atezolizumab” OR “durvalumab” OR “ipilimumab”) AND (“lung cancer” OR “lung tumor” OR “lung neoplasm”). Scientific proceedings from authoritative conference, such as American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO) and World Lung Cancer Conference (WCLC) were also scanned to identify eligible data. We also manually searched the reference lists of the selected articles until no additional potential articles could be identified.
All publications met the following criteria were included: (I) the patients were required to have been diagnosed with advanced lung cancer; (II) randomized control trials that comparing immuno-oncology (IO) combination versus control group (chemotherapy alone/immunotherapy alone); (III) at least one survival data was recorded (PFS or OS). Studies were excluded if they were: (I) reviews, case-only studies, letters, comments or editorials; (II) duplication of previous publications or replicated samples.
Data extraction
Based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (PRISMA) (13) (Supplementary File 1), some items were extracted from the eligible clinical trials as baseline characteristics, including name of the trial, publication year, number of cases, details of combination arm and control arm and PD-L1 testing method. For further subgroup analysis, we extracted survival HRs in the subgroups as followings: age (<65 vs. ≥65 years old), sex (female vs. male), ECOG performance status (0 vs. 1), smoking status (never vs. current/former), liver metastasis (yes vs. no), histology type (non-squamous vs. squamous vs. SCLC), EGFR/ALK status (positive vs. negative), PD-L1 expression (negative vs. weak positive vs. strong positive). Diagnostic antibodies used in the included studies to detect PD-L1 expression varied in different trials, namely 22C3 in Keynote trials, SP142 in IMpower trials and Dako 28-8 in Checkmate trials. However, the standard to define PD-L1 negative and positive remained consistent and was 1% in all trials. Strong positive was defined TPS ≥50% for 22C3 and ≥50% tumor cell (TC) staining and/or ≥10% immune cell (IC) staining for SP142. Therefore, the expression density between strong and negative was defined as PD-L1 weak positive, namely TPS ranging from 1% to 49% detected by 22C3 assay and TC1/2 and/or IC1/2 detected by SP142 assay. Hence, we made subgroup analysis to investigate the association of PD-L1 expression and efficacy of combination strategy in group of PD-L1 negative, weak positive and strong positive expression.
Quality assessment
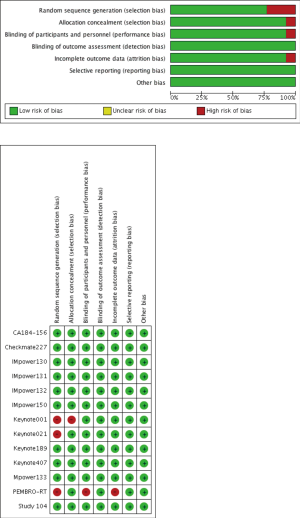
Two reviewers (G Gao and M Qiao) independently evaluated the risk of bias of included studies using the Cochrane Collaboration tool. Details on the risk of bias in thirteen trials were demonstrated in Figure S1.
Statistical analysis
We used generic inverse-variance method to analyze the aggregated survival data. The χ2 test was used to test for statistical significance and I2 test was used to evaluate the heterogeneity across trials. Low-level heterogeneity was interpreted as P>0.1 for the χ2 test and I2 <25%. When there was no statistically significant heterogeneity, fixed-effects model would be used, otherwise, a random-effects model was used. Publication bias was performed by Begg’s funnel plot and the asymmetrical plot indicates the presence of publication bias. Additionally, Egger’s test was used to measure the funnel plot asymmetry on the natural logarithm scale of HR. All data were analyzed by using the Statistical Package for Social Science (SPSS) software (version 23.0 for Mac). Statistical analyses were performed by Revman 5.3.5 (http://tech.cochrane.org/revman) and STATA v12.0 (Stata Corporation, TX, USA). Forest plot to indicate the association between clinicopathological features and efficacy of combination therapy was executed by GraphPad Prism (version 6.0, GraphPad Software, Inc.). P values in this article were two-sided and considered statistically significant when less than 0.05.
Results
Characteristics of eligible studies
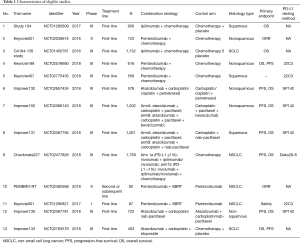
The process of study identification and selection was shown in Figure 1. Finally, a total of 13 clinical trials including 9,241 patients were analyzed in the current meta-analysis (Table 1) (11,14-25). Ten of 13 trials were phase III, 2 of them were phase II and Keynote001 was phase I trial. Anti-PD-1 based immunotherapy were examined in 5 trials and all of them were pembrolizumab-based, namely Keynote021, Keynote189, Keynote407, PEMBRO-RT and Keynote001. Anti-PD-L1 based immunotherapy were examined in 5 trials and all of them were atezolizumab-based, termed IMpower 130, IMpower 131, IMpower 132, IMpower 133 and IMpower 150. Anti-CTLA-4 based drug (ipilimumab) were involved in CA184-156 and Study104. Checkmate 227 was the only IO + IO combination trial. Two clinical trials, IMpower 133 and CA184-156 enrolled SCLC patients. The rest of the studies enrolled mixed type of NSCLC or non-squamous or squamous only. Eight trials provided available data on PD-L1 expression with 3 different diagnostic antibodies (22C3, SP142 and DAKO28-8). Of note, in the case of IMpower150 (arm A), the survival HRs were extracted in both EGFR/ALK-mutated group and EGFR/ALK-wild type group, hence we marked two groups of data as shown in Figure 2 as IMpower (arm A)-WT and IMpower (arm A)-MUT, respectively.
Full table

Progression-free survival and overall survival
13 clinical trials provided PFS data. The pooled results indicated there was significant PFS improvement in combination arm compared with control arm using random-effects model (HR =0.66, 95% CI: 0.59–0.74, P<0.001; I2=70%, P<0.001, Figure 2A). To investigate the heterogeneity between studies, we further performed subgroup analysis. Interestingly, the heterogeneity dramatically decreased when we made subgroup analysis stratified by different type of ICI. As shown in Figure 2B, although patients could get PFS when treated with IO combination, greatest improvement was present in group of PD-1 based combination (HR =0.54, P<0.001; I2=0%, P=0.98), followed by PD-L1 based combination (HR =0.66, P<0.001; I2=11%, P=0.34) and CTLA-4 based combination (HR =0.86, P=0.002; I2=0%, P=0.81) (interaction: P<0.001). Significant heterogeneity still existed between studies in the subgroup of combining IO with chemotherapy even though HRs favored ICI combination (HR =0.67, 95% CI: 0.59–0.75, P<0.001; I2=75%, P<0.001). In the group of IO-radiotherapy combination, fixed effects model was applied and combination did improve PFS compared with control group (HR =0.58, P=0.004; I2=0%, P=0.82) (Figure S2A).
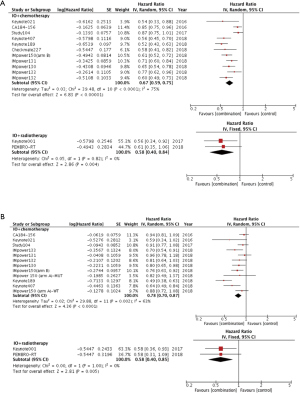
The analysis of OS was based on 12 clinical trials. The aggregated results demonstrated in overall advanced lung cancer patients, OS benefit was evident in combination group using random-effects model (HR =0.77, 95% CI: 0.69–0.86, P<0.001; I2=60%, P=0.002, Figure 2C). Similarly, in the subgroup analysis, PD-1 based combination showed the maximum improvement in OS (HR =0.56, P<0.001; I2=0%, P=0.72) followed by PD-L1 based combination (HR =0.82, P<0.001; I2=0%, P=0.57). In addition, anti-CTLA-4-based combination did not improve OS compared with control group (HR =0.93, P=0.18; I2=0%, P=0.78, Figure 2D). The difference in survival HRs differed by different ICI-based combination (interaction: P<0.001). Irrespective of the partner of combination, patients could benefit from IO combination instead of control group (IO + chemotherapy: HR =0.78, P<0.001; IO + radiotherapy: HR =0.58, P=0.005, Figure S2B).
Impact of PD-L1 expression on efficacy of combination therapy
The data on association of PD-L1 expression and PFS was available in 6 clinical trials on NSCLC patients. As shown in Figure 3A, regardless of PD-L1 expression, patients could benefit from combination therapy based on ICI over chemotherapy. However, the greatest PFS benefit was observed in PD-L1 strong positive group (HR =0.41, 95% CI: 0.34–0.49, P<0.001). Of note, the improvement in PFS with combination versus chemotherapy did differ by PD-L1 expression (negative: HR =0.76, P<0.001; weak positive: HR =0.60, P<0.001; strong positive: HR =0.41, P<0.001; interaction: P<0.001), namely, PFS benefit improved as increasing PD-L1 expression.
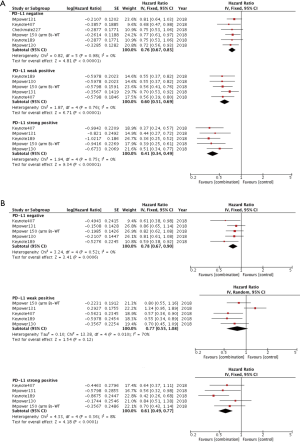
OS data was available in 5 clinical trials as shown in Figure 3B. Patients with negative and strong PD-L1 expression could significantly benefit from combination strategy (negative: HR =0.78, 95% CI: 0.67–0.90, P<0.001; strong positive: HR =0.61, 95% CI: 0.49–0.77, P<0.001) whereas no close correlation was present between combination therapy and OS benefit in the group of patients with weak PD-L1 expression using a random-effects model (HR =0.77, 95% CI: 0.55–1.08, P=0.12; I2=70%, P=0.01).
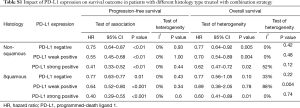
Thereafter, we performed a subgroup analysis stratified by histology type. As shown in Table S1, the combination treatment substantially improve the PFS and OS in non-squamous patients regardless of PD-L1 expression. Interestingly, in squamous patients, although combination treatment had positive effect on PFS, OS benefit could be observed only in patients with strong PD-L1 expression (negative: HR =0.77, P=0.1; weak positive: HR =0.89, P=0.78; strong positive: HR =0.6, P=0.01)
Full table
Subgroup analysis based on clinical characteristics
To explore the impact of clinical characteristics on efficacy of combination strategy versus control group, we performed the subgroup analysis according to age, sex, ECOG PS, smoking status, liver metastasis (LM), histological type and status of driver mutations as shown in Figure 4. Notably, significant PFS benefit of IO combination was observed in all subgroups except in patients with LM (HR =0.83, 95% CI: 0.65–1.07, P=0.14).

However, OS benefit could be mostly observed in male (HR =0.82, P=0.03), current or former smokers (HR =0.74, P=0.04), patients with comparatively worse ECOG performance status (ECOG PS =1, HR =0.74, P=0.009), non-squamous (HR =0.71, P<0.001) and patients without driver mutations (HR =0.73, P<0.001). Whether patients had liver metastasis or not, they all could benefit from ICI based combination (with LM: HR =0.74, P=0.005; without LM: HR =0.66, P<0.001).
Sensitivity analysis and publication bias
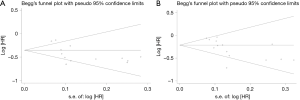
Sensitivity analysis was carried out by deleting one study at one time to detect whether it had influence on pooled HR. In the present meta-analysis, omitting single study did not alter the results which indicated that combination strategy based on ICI could significantly improve the PFS and OS in unselected population (Figure S3). Begg’s test and Egger’s test were both performed to evaluate the publication bias (Figure S4, Table S2). Z value in Begg’s test was 1.04 on PFS (P=0.30) and 1.97 on OS (P=0.05). Egger’s test showed that t score was −2.2 on PFS (P=0.05) and −2.57 on OS (P=0.03). The publication bias for OS was observed. One possible explanation was small sample size in Keynote021 (n=123), Keynote001 (n=97) and PEMBRO-RT (n=92).When we dropped these three studies, Z value in Begg’s test was 1.87 on OS (P=0.062) and t score was −1.94 on OS (P=0.085) (not shown).


Full table
Discussion
The current meta-analysis that comprehensively investigate the association of clinicopathological features and clinical outcome of combination therapy based on ICI in advanced lung cancer patients. Our study highlighted three findings: Firstly, the aggregated results pointed out that combination strategy did significantly improve PFS (HR =0.66, 95% CI: 0.59–0.74, P<0.001) and OS (HR =0.77, 95% CI: 0.69–0.86, P<0.001) compared with control group and the greatest improvement was seen in group of PD-1-based combination (HR =0.54, P<0.001). Secondly, as increasing of PD-L1 expression, the improvement in PFS did proportionally increase (negative: HR =0.76, P<0.001; weak positive: HR =0.60, P<0.001; strong positive: HR =0.41, P<0.001; interaction: P<0.001). OS benefit was not appeared in group of patients with PD-L1 weak-positive group (HR =0.77, 95% CI: 0.55–1.08, P=0.12). Thirdly, subgroup analysis revealed that OS benefit could be observed in male (HR =0.82, P=0.03), current or former smokers (HR =0.74, P=0.04), non-squamous (HR =0.71, P<0.001) and patients without driver mutations (HR =0.73, P<0.001). Patients with LM could get OS benefit instead of PFS benefit from ICI-based combination.
Immunotherapy, especially ICI has shown superior benefit compared with conventional treatment. To benefit larger number of patients and produce durable anti-tumor activity, combining ICI with conventional treatment has come to the stage. Since clinicians had several options on PD-1/PD-L1 inhibitors for patients, how to choose the best one for patients when making combination strategy? An indirect comparison between nivolumab, pembrolizumab and atezolizumab was performed to investigate the best ICI in pre-treated NSCLC (26). It was reported that nivolumab and pembrolizumab were associated with increased of ORR compared with atezolizumab. In this current meta-analysis, PD-1-based combination had the greatest improvement in PFS (HR =0.54, P<0.01) and OS (HR =0.56, P<0.01) compared with PD-L1-based combination (PFS: HR =0.66, P<0.001; HR =0.82, P<0.001). However, CTLA-4-based combination therapy failed to improve OS in this meta-analysis (HR =0.93, 95% CI: 0.83–1.04, P=0.18). The possible biological explanation could be that PD-1 inhibitors, such as nivolumab and pembrolizumab was designed to simultaneously block the ligation between PD-1 and its ligand, PD-L1 and PD-L2; PD-L1 inhibitor, such as atezolizumab was aimed to specifically block the ligation between PD-L1 and PD-1 to restore tumor-specific T-cell immunity (27). However, it was reported that the expression of PD-L2, the other ligand for PD-1 was associated with response to PD-1 axis targeted therapy (28). Compared with wide expression of PD-L1, PD-L2 was restrictedly expressed on antigen-presenting cells (APCs). The interaction between PD-L2 and PD-1 inhibited the T cell proliferation and apoptotic effects of APC cells (29,30). Hence, targeting both PD-1 ligands may produce maximum clinical benefit. When it comes to CTLA-4 inhibitor, the worst clinical outcome showed by the pooled results possibly owing to the limited studies included in this subgroup. Two studies, Study 104 enrolled squamous only and CA184-156 enrolled SCLC patients (14,15). CTLA-4 was the first ICI to be clinically targeted in advanced melanoma (31). It primarily regulated the activated T cells in the priming stage (32). Therefore, even though the early-stage of T cells were activated in the lymphoid compartment, effector T cells localized in the tumor microenvironment might not be effectively stimulated (33). Plus, as patients with squamous or SCLC tended to have high tumor mutational burden, patients were more likely to benefit from PD-1 targeted therapy. Therefore, together with PD-1 inhibitor, promising anti-tumor activity was observed in subgroup analysis in Checkmate227, squamous patients had 36% of 1-year PFS rate treated with nivolumab plus ipilimumab compared with 7% in chemotherapy arm (19). Similarly, encouraging results from Checkmate032 also showed that 2-year OS could be achieved 26% in combination arm (nivolumab + ipilimumab) in patients previously treated with SCLC (9).
To unify a predictive model for ICI is an ultimate goal in the century of immunotherapy. However, although PD-L1 expression was the only predictive biomarker currently used for patients selection, it was still not a satisfying biomarker with several limitations (34,35). Although in our meta-analysis, patients could get PFS benefit across all PD-L1 staining density. Especially, survival HRs became more favorable to combination strategy as the increasing expression of PD-L1. When it comes to OS, the staining density of PD-L1 was not proportionally correlated with clinical efficacy. Greatest improvement in OS was observed in the group of PD-L1 strong positive, whereas, the clinical outcome became heterogeneous across the studies in the group of PD-L1 weak positive. Three of 5 included trials in the group of PD-L1 weak-positive expression was PD-L1 (atezolizumab) based studies, termed IMpower150, IMpower130 and IMpower131 and the results from all the three trial were dismal in this subgroup (18,23,24). Similarly, in OAK and POPLAR, two clinical trials with regard to evaluate the efficacy of atezolizumab versus chemotherapy in treatment of NSCLC in second or subsequent line also showed dismal results in patients with PD-L1 weak positive (5,6). The possible explanation was the diagnostic antibodies varied across the studies, different assay has different ability to make a clear definition of PD-L1 weak positive (36). Another hypothesis was anti-PD-1 inhibitor might truly have superior efficacy compared with PD-L1 inhibitor based combination in patients with negative/low PD-L1 expression. For instance, in Keynote407, squamous patients could get OS benefit from pembrolizumab regardless of PD-L1 expression. However, in IMpower131, no significant OS improvement was observed in squamous patients (HR =0.96, 95% CI: 0.78–1.18, P=0.69). Particularly, only patients with strong positive PD-L1 expression favored combination strategy (HR =0.56, 95% CI: 0.32–0.99). Based on different outcome from two RCTs, Zhang et al. recently mentioned that PD-1 inhibitor plus chemotherapy had better efficacy over PD-L1 based combination, especially in patients with PD-L1 low/negative advanced squamous NSCLC (37). However, more data are anticipated on evaluating the association between PD-L1 expression and efficacy of combination therapy and clarifying whether PD-1 and PD-L1 inhibitor had different efficacy on patients with PD-L1 weak-positive expression.
Our data showed that HRs for OS favored male patients, current/former smokers and patients without EGFR/ALK mutation. It had been postulated that sex hormone had impact on immunomodulation (38). Multiple studies pointed out that advantages of immunotherapy may differ by sex. A recent meta-analysis men derived greater value from immune checkpoint inhibitors compared with women (men: HR = 0.72, 95% CI: 0.65–0.79; women: HR =0.86, 95% CI: 0.79–0.93) (39) which was consistent with our results. However, Wallis presented a conflicting data while performing a meta-analysis included 23 trials across different type of malignancies that the response to ICI did not appear to differ according to sex (40). One possible reason was male patients with lung cancer tended to be smokers compared with other cancers and smokers had comparatively high TMB. It was reported that the average of tumor mutations were 10-fold higher in smokers than non-smokers (41). Additionally, patients who harbored driver mutations had small fraction of nonsynonymous mutations that led to formation of neoantigens that triggered the effective T cell activity (42) and female patients with lung cancer were more likely to have oncogenic mutations. Therefore, the evidence with regard to specific clinicopathological features (male, current or former smokers and patients lacking of specific gene mutations) favored IO combination possibly owing to high TMB. To support this premise, a growing body of evidence showed that tumor mutation burden (TMB) was a potential biomarker that needed to be focused in the future research. In Checkmate026, among patients with a high TMB instead of patients with PD-L1 expression level of at least 5% (HR =1.15, 95% CI: 0.91–1.45, P=0.25) or 50% (HR =1.07, 95% CI: 0.77–1.49), nivolumab was associated with a longer median PFS (9.7 vs. 5.8 months) (43). Both Checkmate568 and Checkmate 227 demonstrated robust antitumor activity was observed in patients treated with nivolumab and ipilimumab with TMB of 10 or more mutations per megabase irrespective of PD-L1 expression (19,44). However, there was no standard to define high TMB with determined threshold and unified NGS panels. In addition, as data shown in IMpower133, patients with both above and below the prespecified cutoffs of 10 and 16 mutations per megabase could benefit from combination group (21). Therefore, prospective evaluation of TMB as a biomarker with proper cut-off is eagerly awaited. Furthermore, previous evidence pointed out STK11 loss was associated with poor efficacy of pembrolizumab in combination with chemotherapy and concurrent mutations in STK11 and KEAP1 was correlated to resistance to ICI blockade in NSCLC patients with high TMB (45). Taken together, predictive markers for combination were complex and it was critical to establish a model that mix valuable factors to comprehensively evaluate and select the potential population for treatment with IO combination (46). Additionally, before taking into specific clinicopathological features account in clinical practice, clinicians must adjust for underlying genetic and protein markers to make better decision (47,48).
Liver metastasis is a negative biomarker among NSCLC. The liver is a tolerogenic organ with unique immune regulation to guarantee the local and systemic immune tolerance which is favorable in the setting of organ transplantation. However, this characteristic is detrimental in fighting against cancer. Despite it was the primary site of T-cell activation, it elicited mostly poor and incomplete activation, even suppression and exhaustion (49). Previous studies reported that liver metastasis was associated with PFS and a lower response rate in nivolumab-treated and pembrolizumab-treated NSCLC patients (50). Additionally, reduced CD8+T cell density was observed at the invasion margin of tumor (51). In the present study, we found that patients with LM could not get PFS benefit but OS benefit from IO combination. Obvious benefit was observed in subgroup analysis of IMpower150-armB. OS was significantly prolonged in patients with liver metastasis compared with patients without liver metastasis treated with atezolizumab plus bevacizumab and chemotherapy. However, dismal results were observed in armA which did not apply additional bevacizumab. Similarly, OS benefit was not observed in IMpower132 and IMpower131 which experimental arm was combining ICI and chemotherapy. Since multi-kinase angiogenesis inhibitors were standard therapy in patents with advanced hepatocellular carcinoma (HCC) (52), does anti-angiogenesis also play an irreplaceable role in the treatment of NSCLC patients with LM? Will additional anti-angiogenesis therapy bring about robust anti-tumor activity? Additionally, updated data from Keynote189 in 2019 American Association for Cancer research (AACR) showed that prolonged OS was also observed in patients with liver metastasis treated with pembrolizumab-based combination. Whether patients with liver metastasis could truly benefit from IO combination required more prospective data and preclinical evidence.
There are several meta-analysis that investigated the safety and efficiency of ICI for lung cancer (53-55). However, this study integrated survival data from 13 RCTs with updated clinical data regarding the combination therapy based on ICI and comprehensively analyzed the association between clinicopathological features and efficacy of ICIs plus conventional treatment. The findings provided new insight on (I) choosing the best IO agent when making combination combo; (II) re-evaluating the role of PD-L1 expression in IO combination, especially in patients with weak-positive expression; (III) re-thinking of the underlying biomarkers via analyzing characteristics of patients who had OS benefit from combination. However, this study had several limitations that had to be acknowledged: (I) The number of studies included in the subgroup analysis was comparatively small. For instance, only two studies provided available data on PFS and OS in subgroup of CTLA-4-based combination. (II) The OS data had high heterogeneity owing to some small sample size studies. (III) Owing to the different diagnostic efficacy of different antibodies detecting PD-L1 expression, it might be bias to analyze the association of PD-L1 staining density and clinical outcome. (IV) Clinical characteristics were only prompt to investigate the underlying mechanisms instead of simply being a predictive biomarker. For instance, we did not find prolonged OS [HR =0.69 (95% CI: 0.58–0.82); P<0.001] in patients with good performance (ECOG PS =0, HR =0.78, 95% CI: 0.53–1.16, P=0.22). Confounding factors, such as PD-L1 expression, metastatic status, genetic aberrations were points of consideration. (V) Since immune-related adverse effects are also parts of concern in the application of combination strategy. We did not include this part of analysis.
Conclusions
In conclusion, the present study indicated that IO combination did improve OS and PFS compared with control group. PD-1based combination led to the greatest PFS and OS improvement. The improvement in PFS with combination did proportionally differ by PD-L1 expression. More data are warranted to address the association of PD-L1 staining intensity with OS improvement and to investigate which is the best agent in combination combo for PD-L1 weak-positive expression. Subgroup analysis showed that male, current/former smokers, non-squamous, patients without driver mutations did benefit from combination strategy. Whether patients with liver metastasis could truly benefit from IO combination needed further investigation.
Supplementary
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81672286), National R&D projects (2016YFC0902300), Shanghai Science and Technology Medical Guidance Project (16411964400).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Qiao M, Jiang T, Ren S, et al. Combination Strategies on the Basis of Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer: Where Do We Stand? Clin Lung Cancer 2018;19:1-11. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2018;29:84-91. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Govindan R, Szczesna A, Ahn MJ, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3449-57. [Crossref] [PubMed]
- Reck M, Luft A, Szczesna A, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:3740-8. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Barlesi F, Nishio M, Cobo M, et al. IMpower132: Efficacy of atezolizumab (atezo) + carboplatin (carbo)/cisplatin (cis) + pemetrexed (pem) as 1L treatment in key subgroups with stage IV non-squamous non-small cell lung cancer (NSCLC). ESMO 2018;Abstract LBA65.
- Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36:LBA9000. [Crossref]
- Cappuzzo F, McCleod M, Hussein M. IMpower130: progression-free survival (PFS) and safety analysis from a randomised phase 3 study of carboplatin + nab-paclitaxel (CnP) with or without atezolizumab (atezo) as first-line (1L) therapy in advanced non-squamous NSCLC. ESMO 2018;Abstract LBA53.
- Theelen W, Lalezari F, Vries J, et al. Randomized phase II study of pembrolizumab after SBRT versus pembrolizumab alone in patients with advanced non-small cell lung cancer: The PEMBRO-RT study. J Clin Oncol 2018;36:Abstr 9023.
- Passiglia F, Galvano A, Rizzo S, et al. Looking for the best immune-checkpoint inhibitor in pre-treated NSCLC patients: An indirect comparison between nivolumab, pembrolizumab and atezolizumab. Int J Cancer 2018;142:1277-84. [Crossref] [PubMed]
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer 2015;112:1421-7. [Crossref] [PubMed]
- Yearley JH, Gibson C, Yu N, et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin Cancer Res 2017;23:3158-67. [Crossref] [PubMed]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. [Crossref] [PubMed]
- Rodig N, Ryan T, Allen JA, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol 2003;33:3117-26. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994;1:405-13. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer 2016;4:48. [Crossref] [PubMed]
- Steuer CE, Ramalingam SS. Tumor Mutation Burden: Leading Immunotherapy to the Era of Precision Medicine? J Clin Oncol 2018;36:631-2. [Crossref] [PubMed]
- Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 2018;13:1302-11. [Crossref] [PubMed]
- Zhang Y, Zhou H, Zhang L. Which is the optimal immunotherapy for advanced squamous non-small-cell lung cancer in combination with chemotherapy: anti-PD-1 or anti-PD-L1? J Immunother Cancer 2018;6:135. [Crossref] [PubMed]
- Polanczyk MJ, Hopke C, Vandenbark AA, et al. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res 2006;84:370-8. [Crossref] [PubMed]
- Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol 2018;19:737-46. [Crossref] [PubMed]
- Wallis CJD, Butaney M, Satkunasivam R, et al. Association of Patient Sex With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-analysis. JAMA Oncol 2019;5:529-36. [Crossref] [PubMed]
- Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121-34. [Crossref] [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Ready N, Hellmann MD, Awad MM, et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol 2019;37:992-1000. [Crossref] [PubMed]
- Skoulidis F, Elamin Y, Lam V, et al. Impact of STK11/LKB1 Genomic Alterations on Clinical Outcomes with Chemo-Immunotherapy in Non-Squamous NSCLC. J Thorac Oncol 2018;13:S424-5. [Crossref]
- Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019;19:133-50. [Crossref] [PubMed]
- Abdel-Rahman O. Does a patient's sex predict the efficacy of cancer immunotherapy? Lancet Oncol 2018;19:716-7. [Crossref] [PubMed]
- Ulrich BC, Guibert N. Immunotherapy efficacy and gender: discovery in precision medicine. Transl Lung Cancer Res 2018;7:S211-3. [Crossref] [PubMed]
- Horst AK, Neumann K, Diehl L, et al. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol 2016;13:277-92. [Crossref] [PubMed]
- Shiroyama T, Suzuki H, Tamiya M, et al. Clinical Characteristics of Liver Metastasis in Nivolumab-treated Patients with Non-small Cell Lung Cancer. Anticancer Res 2018;38:4723-9. [Crossref] [PubMed]
- Tumeh PC, Hellmann MD, Hamid O, et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res 2017;5:417-24. [Crossref] [PubMed]
- Lin YY, Tan CT, Chen CW, et al. Immunomodulatory Effects of Current Targeted Therapies on Hepatocellular Carcinoma: Implication for the Future of Immunotherapy. Semin Liver Dis 2018;38:379-88. [Crossref] [PubMed]
- Wu Y, Shi H, Jiang M, et al. The clinical value of combination of immune checkpoint inhibitors in cancer patients: A meta-analysis of efficacy and safety. Int J Cancer 2017;141:2562-70. [Crossref] [PubMed]
- Huang Q, Zhang H, Hai J, et al. Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: A meta-analysis of randomized trials. Oncoimmunology 2018;7:e1396403. [Crossref] [PubMed]
- Xu X, Huang Z, Zheng L, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies combined with chemotherapy or CTLA4 antibody as a first-line treatment for advanced lung cancer. Int J Cancer 2018;142:2344-54. [Crossref] [PubMed]