
Prognostic significance of IMP-3 expression pattern in esophageal squamous cell carcinoma
Introduction
Esophageal cancer is one of the most malignant gastroenterological cancers, with a 5-year survival rate after surgery of 54.5% for all stages, 38.3% for cStage III, 23.6% for cStage IVA, and 18.2% for cStage IVB (1). Pathologically, squamous cell carcinoma accounts for about 90% of all esophageal cancers in Japan.
To improve treatment strategies for patients with esophageal squamous cell carcinoma (ESCC), a biomarker predicting the malignant potential of cancer cell metastasis to lymph nodes and distant organs and the efficacy of treatments including surgery, chemotherapy, and radiotherapy is needed. Various kinds of prognostic biomarkers are known to exist, including VEGF and the vasohibin family (angiogenesis); EGFR, cyclin D1, Ki67, p53 and p16 (replicative potential); E-cadherin and the laminin-5 gamma-2 chain (invasion and metastasis); and squamous cell carcinoma antigen (SCC-antigen; serum marker) (2-10). Since EGFR and VEGF are thought to be good targets for molecular targeting therapy and several monoclonal antibodies have actually been used for the treatment of lung cancer and colorectal cancer, new biomarkers should continue to be investigated for not only diagnosis, but also the development of treatments including molecular targeting treatments (11,12).
The human insulin-like growth factor II m-RNA-binding protein 3 (IMP3) is a member of the RNA-binding protein family, which plays important roles in cell growth, cell migration, trafficking and stabilization during the early stages of embryogenesis. IMP3, which is also known as KOC (KH domain containing protein overexpressed in cancer cells), is encoded by a 4,158-nucleotide RNA transcript, resulting in a protein of 580 amino acids. The gene is located at chromosome 7p11.5, a locus frequently amplified in multiple cancers (13-17). IMP3 is reportedly overexpressed in gastrointestinal cancers including ESCC, urologic cancers, ovarian cancers, and lung cancers, and a high IMP3 expression level is associated with a poor prognosis in patients with those cancers (18). A phase II clinical trial of immune-therapy using peptides derived from ideal cancer-testis antigens including IMP3, LY6K, and CDCA1 for the treatment of esophageal squamous cell cancer has been performed (19). Therefore, such immunotherapy using the IMP3 molecule is promising (20). However, the clinical significance of the IMP-3 expression pattern in the tumor remains unclear.
To the best of our knowledge, this is the first study to analyze the IMP-3 expression pattern in ESCC in detail. The aim of this study was to investigate the relationship between the IMP3 expression pattern in ESCC tumors and the outcomes of patients with ESCC.
Methods
Patients
One hundred and seventy patients with ESCC who underwent radical surgery between 2003 and 2005 at Tokai University Hospital (Isehara, Kanagawa, Japan) were investigated. A transthoracic esophagectomy and three-field lymphadenectomy were performed as standard surgical techniques during this period. Node-positive patients received adjuvant chemotherapy with cisplatin (CDDP) and 5-fluorouracil (5-FU). We excluded patients with synchronous or metachronous multi-organ primary cancers and tissue types other than squamous cell cancer. The patients were followed up using endoscopy, computed tomography (CT), ultrasonography (US), and blood tests including tumor marker, SCC and CEA levels, every 6 months for 5 years after surgery. The esophageal cancers were mainly classified according to the Japanese Classification of Esophageal Cancer (21,22). This study was approved by the institutional review board of Tokai University Hospital, Isehara, Japan (registration No. 13R-058). The need for written informed consent was waived due to the retrospective, non-interventional nature of the present study.
Immunohistochemical staining
The surgically resected tumor specimens and metastatic lymph nodes were fixed in 10% formalin for 24 hours and embedded in paraffin. Four-micrometer-thick paraffin sections were mounted on silane-coated glass slides and deparaffinized in xylene (5 minutes, 3 times) and ethyl alcohol (3 minutes, 4 times). Antigen retrieval was performed using the following process. After washing with 0.01-M phosphate buffered saline (PBS), the slides were incubated in 0.01-M Tris-buffered saline at 98 °C for 20 minutes and then left at room temperature for 60 minutes. After washing with 0.01-M PBS once again, the endogenous peroxidase activity was abolished in 0.3% H2O2 in methanol for 30 minutes. This reaction was then blocked with 10% normal sheep serum for 10 minutes, and the slides were incubated with a mouse monoclonal anti-human IMP3 antibody (Dako Cytomation, Glostrup, Denmark) at 4 °C overnight. Biotinylated anti-mouse IgG antibody (Vectastain ABC Kit, Burlingame, CA, USA) was used as the second antibody. After washing with 0.01-M PBS, the labeled antigen was visualized using the diaminobenzidine reaction. The sections were counterstained with hematoxylin. The placenta was used as a positive control. Cancerous tissue from an esophageal cancer was used as a negative control after the addition of 0.01-M PBS instead of a mouse monoclonal anti-human IMP3 antibody.
Expression pattern of IMP3
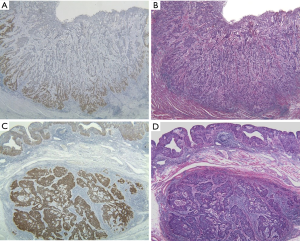
The positive expression of IMP3 was detected when brown granules were identified in the cytoplasm and more than 10% of the cancer cells in each section were immunoreactive to IMP3. The immunohistochemical staining results were assessed by two independent investigators with no knowledge of the clinicopathological data. Although the interpretations of the expression patterns differed between the two pathologists in 21 (12.4%) of the 170 cases, a final decision was made after a review and discussion. The characteristics of the cancer cell staining pattern were classified into two expression patterns: an invasive front-type (IF-type) characterized by intense staining at the tumor IF, and a diffuse-type (D-type) characterized by diffuse staining throughout the whole tumor (Figure 1).
Statistical analysis
The differences in clinicopathological factors between IMP3-positive and IMP3-negative patients and between IF-type patients and D-type patients were analyzed using a chi-square test and an unpaired t-test. The Cox proportional hazard regression model was used to analyze the independent prognostic factors using univariate and multivariate analyses. Variables showing a univariate association (P<0.10) were included in a multivariate analysis. The survival rates were calculated using the Kaplan-Meier method, and the two groups were compared using a log-rank test. Statistical differences were considered significant for P<0.05. All the analyses were performed using the statistical software package IBM SPSS Statistics ver.25.0 (IBM Japan, Tokyo, Japan).
Results
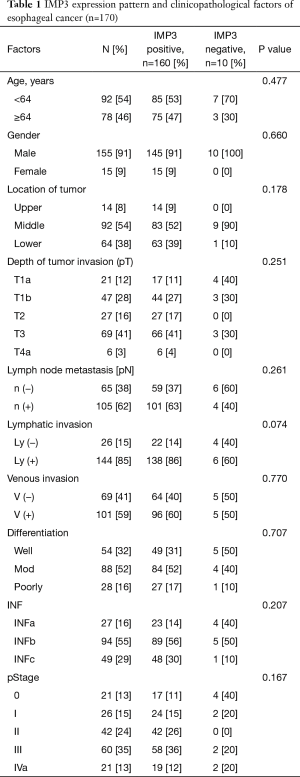
Of the 170 patients, 160 patients (94%) were IMP3-positive in the cytoplasm of their cancer cells (IMP3-positive group), and 10 patients (6%) were IMP3-negative (IMP3-negative group) (Figure 1). The background data for the clinicopathological factors in both groups are shown in Table 1. There was no correlation between IMP3 expression and the clinicopathological factors (Table 1).
Full table
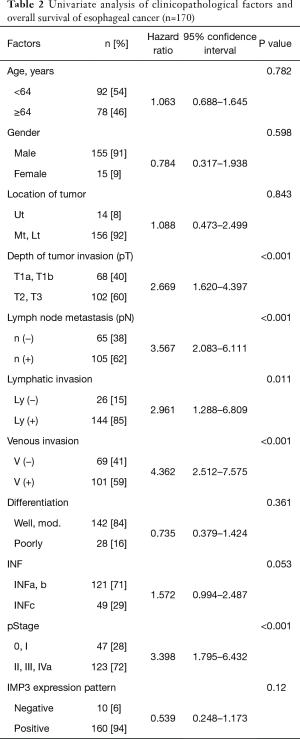
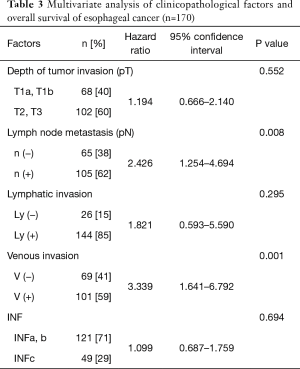
In the survival analysis, univariate analyses revealed that a deeper pT factor (HR =2.669, P<0.001), positive lymph node metastasis (HR =3.567, P<0.001), positive lymphatic invasion (HR =2.961, P=0.011), positive venous invasion (HR =4.362, P<0.001), and the pStage (HR =3.398, P<0.001) were prognostic factors (Table 2). A multivariate analysis also showed that positive lymph node metastasis (HR =2.426, P=0.008) and venous invasion (HR =3.339, P=0.001) were prognostic factors (Table 3). There was no significant difference in the overall survival curves between the IMP3-positive group and the IMP3-negative group (P=0.114) (Figure 2).
Full table
Full table
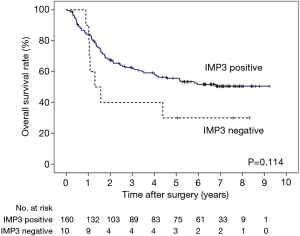
Most of the patients (94%) were IMP3-positive, and IMP3 positivity was not related to the prognostic value. Therefore, when the survival analysis was confined to the 160 IMP3-positive patients, the IMP3 expression pattern was IF-type in 46 patients (29%) and D-type in 114 patients (71%).
IF-type IMP3 expression was related to a deeper pT factor (P=0.024), positive lymph node metastasis (P=0.012), positive venous invasion (P<0.001) and pStage (P=0.001) (Table 4).
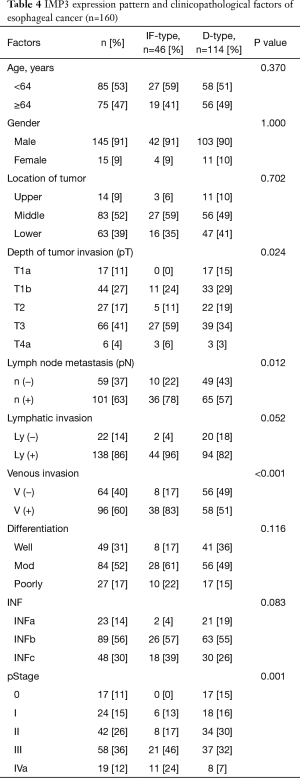
Full table
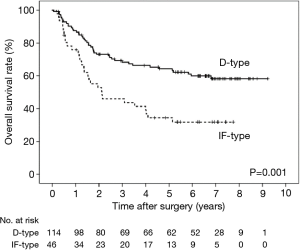
Univariate analyses of overall survival revealed that a deeper pT factor (HR =2.933, P<0.001), positive lymph node metastasis (HR =3.845, P<0.001), positive lymphatic invasion (HR =3.840, P=0.009), positive venous invasion (HR =4.518, P<0.001), INFc (HR =1.621, P=0.046), pStage (HR =4.024, P<0.001), and an IF-type IMP3 expression pattern (HR =2.221, P=0.001) were prognostic factors (Table 5). A multivariate analysis also showed that positive lymph node metastasis (HR =2.489, P=0.009), positive venous invasion (HR =2.749, P=0.006) and an IF-type IMP3 expression pattern (HR =1.618, P=0.049) were prognostic factors (Table 6). The overall survival curve for the IF-type group was significantly worse than that of the D-type group (P=0.001) (Figure 3).
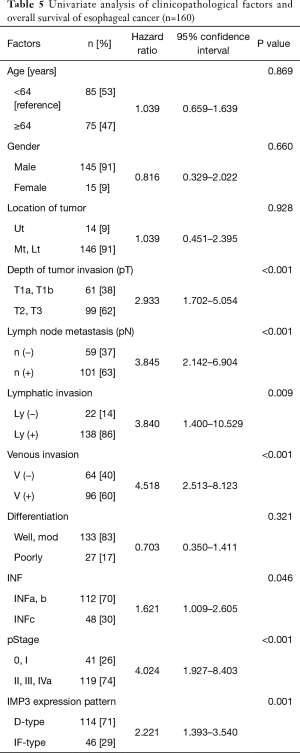
Full table
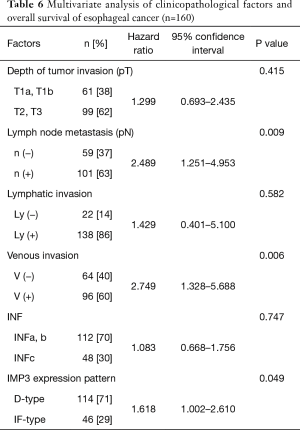
Full table
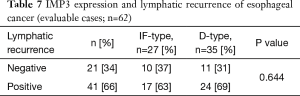
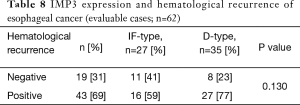
As for the pattern of cancer recurrence in the patients, 62 patients died of the primary disease, 41 patients developed lymph node recurrence, 43 patients developed hematogenous metastasis, and 23 patients developed concurrent lymph node-hematogenous metastases. The site of hematogenous metastatic involvement was the liver in 21 patients, the lung in 18 patients, the bone in 8 patients, the pleura in 8 patients, the skin in 4 patients, and the brain in one patient. The IMP3 expression pattern was not correlated with the type of recurrence (lymph node recurrence, P=0.644, hematogenous metastasis P=0.130) (Tables 7,8).
Full table
Full table
Discussion
In this study, IMP3 was expressed in a large proportion (94%) of ESCC cases, and IMP3 was not expressed in only 10 cases (6%). Therefore, no conclusions can be made regarding the relation between the presence or absence of IMP3 expression and patient outcome until a larger number of cases have been examined. However, when the expression patterns in the tumor tissue were analyzed in detail, they could be classified into two types of patterns: an IF-type (29%), and a diffuse-type (D-type) (71%). A multivariate analysis showed that an IF-type IMP3 expression pattern was a significant predictor of a poor outcome.
IMP3 is reportedly over-expressed in many cancer cells, including esophageal cancer (18), as well as some normal cells, including testicular cells in adults and placenta cells. Therefore, IMP-3 is considered to be an oncofetal protein. The IMP3 expression rate varies according to the type of cancer; for example, the expression rate is reportedly 54.5–70.5% for oral cancer, 59.2% for esophageal cancer, 74.0–81.5% for gastric cancer, 34.9–76.9% for colorectal cancer, 18.4–70.7% for hepatocellular carcinoma (HCC), 53.1–63.0% for pancreatic cancer, 32.4–74.7% for lung cancer, 12.6–51.9% for renal cell carcinoma, 12.2–26.9% for urothelial carcinoma, 18.1–83.8% for prostate cancer, and 47.1–63.0% for ovarian cancer. Previous reports have suggested that IMP3 contributes to various aspects of cancer by promoting the expressions of target genes either by preventing mRNA decay or by stimulating mRNA translation (18). The role of IMP3 in cancer cells remains controversial; however, numerous reports have suggested that IMP3 promotes tumor cell invasion and migration by targeting epithelial-mesenchymal transition-associated molecular markers including E-cadherin, Slug and vimentin (23).
In this study, IMP3 was expressed in 94% of the esophageal cancers, and IMP3 expression was related to neither clinicopathological factors nor overall survival. The outcomes of patients with a high expression of IMP3 are poorer than those of patients without a high expression of IMP3 in many kinds of cancer. Various criteria for high IMP3 expression exist; for instance, values of more than 0%, 5%, 10%, or 50% positivity have been reported as high IMP3 expression (18). A value of 0% positivity was chosen for this study and a value of 10% positivity was chosen for a previous report on esophageal cancer (24). This was the reason why the positive rates of IMP3 expression were 94% in this study and 59.2% in the previous study.
In this study, when simple criteria for positivity and negativity were adopted, no clinically significant difference in the IMP3 expression status was seen. After a detailed analysis of the IMP3 expression pattern, however, we found two types of patterns: an IF-type and a D-type. Based on these expression patterns, the patients were divided into two groups: patients with IF-type IMP3 expression, and those with D-type IMP3 expression.
Cancer cells in IF-type tumors seem to be more aggressive than cancer cells in D-type tumors. The reason for this phenomenon is difficult to explain. However, we were able to refer to a study on HCC (25). In HCC tumors, multiple IMP3 expression patterns have been described: diffuse positivity (33%), heterogeneous to focal positivity (28%), and positivity in a small number of tumor cells (39%). In tumors with heterogeneous to focal positivity and positivity in a small number of tumor cells, IMP3 was predominantly expressed at the peripheries of the tumor nest, at the IF, and in satellite nodules. The existence of several kinds of IMP3 expression patterns in HCC tumors was similar to that seen in esophageal cancer in the present study. The previous report suggested that high mobility group A2 (HMGA2), which is an oncofetal protein involved in cell proliferation, neoplastic transformation, and tumor invasion, also tended to be expressed at the tumor periphery and IF. Strong staining for HMGA2 was also reportedly observed at the IF of gastric cancer (26) and in oral squamous cell carcinoma (27). Moreover, Kuwano et al. suggested that cancer cell proliferation of ESCC was the main mechanism of tumor progression at the invasive site of tumors (28). These reports supported our finding that IF-type tumors were more aggressive than D-type tumors, and patients with IF-type tumors might have a poor prognosis, however, these mechanisms have not yet been adequately investigated.
The first limitation of this study was its retrospective design. Second, in this study, only 6% of the examined cases were negative for IMP3, while 94% were IMP3-positive; we mainly focused on the IMP3-positive cases and analyzed them in terms of the IMP3 expression pattern. In a future study, we would like to increase the sample size and to analyze the clinical significance of both positive and negative IMP3 expression patterns. Third, like the HCC report, a detailed study is needed to clarify which cancer cells in IF-type tumor have aggressive potential.
To our knowledge, this study is the first report to suggest that an IF-type IMP3 expression pattern is a predictor of a poor prognosis in patients with ESCC. The relationship between the IMP3 expression pattern in ESCC and the efficacy of peptide vaccine therapy using IMP3 should be examined in the future.
Acknowledgments
The authors would like to thank Ms. Makiko Tanaka (Department of Gastroenterological Surgery, Tokai University School of Medicine) for support with the immunohistochemical staining and Ms. Izu Inada (Department of Gastroenterological Surgery, Tokai University School of Medicine) for support with the data analysis. The authors used an English Language Service (International Medical Information Center, Tokyo, Japan) for language editing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board of Tokai University Hospital, Isehara, Japan (registration No. 13R-058). The need for written informed consent was waived due to the retrospective, non-interventional nature of the present study
References
- Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive Registry of Esophageal Cancer in Japan, 2011. Esophagus 2018;15:127-52. [Crossref] [PubMed]
- Chen M, Huang J, Zhu Z, et al. Systematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancer. BMC Cancer 2013;13:539. [Crossref] [PubMed]
- Shih CH, Ozawa S, Ando N, et al. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res 2000;6:1161-8. [PubMed]
- Ninomiya Y, Ozawa S, Oguma J, et al. Expression of vasohibin-1 and -2 predicts poor prognosis among patients with squamous cell carcinoma of the esophagus. Oncology Letters 2018;16:5265-74. [PubMed]
- Ozawa S, Ueda M, Ando N, et al. Prognostic significance of epidermal growth factor receptor in esophageal squamous cell carcinomas. Cancer 1989;63:2169-73. [Crossref] [PubMed]
- Kitagawa Y, Ueda M, Ando N, et al. Further evidence for prognostic significance of epidermal growth factor receptor gene amplification in patients with esophageal squamous cell carcinoma. Clin Cancer Res 1996;2:909-14. [PubMed]
- Shinozaki H, Ozawa S, Ando N, et al. Cyclin D1 amplification as a new predictive classification for squamous cell carcinoma of the esophagus, adding gene information. Clin Cancer Res 1996;2:1155-61. [PubMed]
- Takeuchi H, Ozawa S, Ando N, et al. Altered p16/MTS1/CDKN2 and cyclin D1/PRAD-1 gene expression is associated with the prognosis of squamous cell carcinoma of the esophagus. Clin Cancer Res 1997;3:2229-36. [PubMed]
- Ito E, Ozawa S, Kijima H, et al. Clinicopathological significance of laminin-5γ2 chain expression in superficial esophageal cancer. Dis Esophagus 2014;27:463-9. [Crossref] [PubMed]
- Oguma J, Ozawa S, Kazuno A, et al. Wnt3a expression is associated with poor prognosis of esophageal squamous cell carcinoma. Oncol Lett 2018;15:3100-8. [PubMed]
- Yamaoka T, Ohba M, Ohmori T. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. Int J Mol Sci 2017;18:E2420. [Crossref] [PubMed]
- Maj E, Papiernik D, Wietrzyk J. Antiangiogenic cancer treatment: The great discovery and greater complexity Int J Oncol 2016;49:1773-84. (Review). [Crossref] [PubMed]
- Müeller-Pillasch F, Lacher U, Wallrapp C, et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 1997;14:2729-33. [Crossref] [PubMed]
- Mueller-Pillasch F, Pohl B, Wilda M, et al. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech Dev 1999;88:95-9. [Crossref] [PubMed]
- Nielsen FC, Nielsen J, Christiansen J. A family of IGF-II mRNA binding proteins (IMP) involved in RNA trafficking. Scand J Clin Lab Invest Suppl 2001;234:93-9. [Crossref] [PubMed]
- Vikesaa J, Hansen TVO, Jonson L, et al. RNA-binding IMPs promote cell adhesion and invadopodia formation. EMBO J 2006;25:1456-68. [Crossref] [PubMed]
- Rivera Vargas T, Boudoukha S, Simon A, et al. Post-transcriptional regulation of cyclins D1, D3 and G1 and proliferation of human cancer cells depend on IMP-3 nuclear localization. Oncogene 2014;33:2866-75. [Crossref] [PubMed]
- Chen L, Xie Y, Li X, et al. Prognostic value of high IMP3 expression in solid tumors: a meta-analysis. Onco Targets Ther 2017;10:2849-63. [Crossref] [PubMed]
- Kono K, Iinuma H, Akutsu Y, et al. Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens. J Transl Med 2012;10:141. [Crossref] [PubMed]
- Ishikawa N, Takano A, Yasui W, et al. Cancer-testis antigen lymphocyte antigen 6 complex locus K is a serologic biomarker and therapeutic target for lung and esophageal carcinomas. Cancer Res 2007;67:11601-11. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:1-36.
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus 2017;14:37-65.
- Su P, Hu J, Zhang H, et al. IMP3 expression is associated with epithelial-mesenchymal transition in breast cancer. Int J Clin Exp Pathol 2014;7:3008-17. [PubMed]
- Takata A, Takiguchi S, Okada K, et al. Expression of insulin-like growth factor-II mRNA-binding protein-3 as a marker for predicting clinical outcome in patients with esophageal squamous cell carcinoma. Oncol Lett 2014;8:2027-31. [Crossref] [PubMed]
- Jeng YM, Chang CC, Hu FC, et al. RNA-binding protein insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology 2008;48:1118-27. [Crossref] [PubMed]
- Motoyama K, Inoue H, Nakamura Y, et al. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clin Cancer Res 2008;14:2334-40. [Crossref] [PubMed]
- Miyazawa J, Mitoro A, Kawashiri S, et al. Expression of mesenchyme-specific gene HMGA2 in squamous cell carcinoma of the oral cavity. Cancer Res 2004;64:2024-9. [Crossref] [PubMed]
- Kuwano H, Saeki H, Kawaguchi H, et al. Proliferative activity of cancer cells in front and cancer areas of carcinoma in situ and invasive sites of esophageal squamous-cell carcinoma. Int J Cancer 1998;78:149-52. [Crossref] [PubMed]


