
Prognostic impact of the Controlling Nutritional Status score in patients with non-small cell lung cancer treated with pembrolizumab
Introduction
Non-small cell lung cancer (NSCLC) is the most common cause of cancer related death worldwide, accounting for more than one million deaths annually. In the past, traditional cytotoxic chemotherapy was the only treatment for unresectable advanced or recurrent NSCLC. Recently, various molecular target drugs, such as epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have been approved for the treatment of advanced NSCLC (1) and have played an important role in the patients with specific mutations. Furthermore, immunotherapy using agents such as immune-checkpoint inhibitors (ICI) has been a focus of attention (2) and their effectiveness in the treatment of NSCLC has been reported (3-8). The emergence of these therapeutic agents has greatly advanced the treatment of lung cancer.
The first ICI to show effectiveness in the treatment of NSCLC was nivolumab, a programmed cell death-1 (PD-1) inhibitor. Nivolumab prolonged the overall survival (OS) compared with standard second-line docetaxel treatment in two independent phase III studies in previously treated patients with advanced squamous (CheckMate 017) or nonsquamous (CheckMate 057) NSCLC (3,4). The expression of the PD-1 ligand (PD-L1) was neither prognostic nor predictive of benefit in these trials. Recently, the PD-L1 inhibitor atezolizumab has also been shown to prolong the OS compared to standard second-line docetaxel treatment in previously treated NSCLC, regardless of the PD-L1 expression or histology, with a favorable safety profile (OAK trial) (6). Pembrolizumab, another PD-1 inhibitor, significantly prolonged the progression-free survival (PFS) and OS in locally advanced or metastatic NSCLC patients who were previously untreated compared to the conventional standard platinum combination therapy. A prognostic benefit was seen in patients with PD-L1 expression ≥50% (7). Recently, even in NSCLC with PD-L1 expression of 1–50%, the effects of pembrolizumab have been comparable to with fewer adverse events than chemotherapy (8). Furthermore, pembrolizumab prolonged the OS in previously treated NSCLC patients with PD-L1-positive cells >1% compared to the standard second-line docetaxel treatment (5).
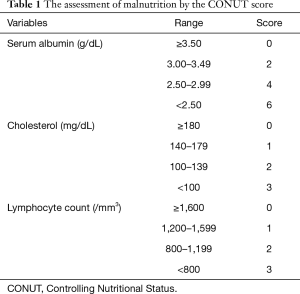
The importance of the patient’s immunonutritional status in cancer treatment is well known (9,10). The relationship between the neutrophil-to-lymphocyte ratio (NLR), which is a commonly used index, and the effect of ICI therapy has recently been reported in NSCLC patients (11,12). The Controlling Nutritional Status (CONUT), a novel nutritional index, is determined based on the serum levels of albumin and cholesterol, and the lymphocyte count (Table 1). The CONUT is a simple and useful method for evaluating a patient’s nutritional status, and it has also been reported to be highly reliable and to correspond to the results of standard nutritional assessments (13,14). Recently, the relationship between the CONUT score and the perioperative risk and postoperative prognosis has been reported in various cancers, including urothelial carcinoma, liver cancer, cholangiocarcinoma, gastric cancer and lung cancer (15-19). We reported that the CONUT score is an independent predictor of the effectiveness of treatment and the prognosis of patients with malignant pleural mesothelioma (MPM) (20). Several studies have shown the relevance of the CONUT score in metastatic cancer patients undergoing chemotherapy treatment; however, to our knowledge, none has examined its usefulness in patients undergoing ICI treatment (20-22).
Full table
The present study aims to clarify the clinical significance of the CONUT score in NSCLC patients treated with pembrolizumab.
Methods
Patients
From February 2017 to January 2018, 49 NSCLC patients started pembrolizumab treatment (200 mg/body, intravenously every 3 weeks) at the clinical research institute, National Hospital Organization Kyushu Cancer Center (Fukuoka, Japan). In all cases a diagnosis of unresectable or postoperative recurrent stage III or IV NSCLC was histologically confirmed. Seventeen patients who received pembrolizumab as a second-line or later treatment were excluded because their serum cholesterol data were unavailable. Thus, the data of 32 patients were analyzed in the present study.
Data collection
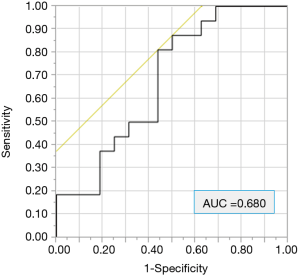
The following data were collected: age, sex, ECOG performance status (PS), smoking history [light smoker, pack year index (PYI) <20; heavy smoker, PYI ≥20], tumor histology, and the PD-L1 expression status. We also evaluated the NLR. The receiver operating characteristic (ROC) curve for 9-month PFS revealed that the optimal cut-off NLR score was 4.11 (Figure 1). In the evaluation of the CONUT score, the albumin and cholesterol levels and the lymphocyte count were investigated in blood examinations within one month before treatment. The CONUT score was defined as previously described (Table 1) (20,23,24). We used a cut-off CONUT score of “2”, based on previous reports (20,23,24), and classified patients with a CONUT score of ≤2 into the CONUT low group, and those with a score of >2 into the CONUT high group. Follow-up data were collected until October 15, 2018 or the date of death. The median follow-up period was 12.0 months.
The tumor PD-L1 protein expression level was examined in archived formalin-fixed and paraffin-embedded tumor biopsy samples. Immunohistochemical staining of PD-L1 was conducted using the PD-L1 IHC 22C3 pharmDx antibody (clone 22C3, Dako North America, Inc., Agilent/Dako, Carpinteria, CA, USA) according to methods recommended for the detection of DAKO (23), in accordance with the manufacturer’s protocol. Stained slides were independently scored by at least two observers, including a well-trained certified pathologist. According to the kit manufacturer’s criteria, cases in which the membrane was positively stained in ≥50% of the tumor cells were defined as positive. PD-L1 positivity was defined by a positive tumor proportion score (TPS) of ≥50%.
The National Hospital Organization Kyushu Cancer Center Institutional Review Board approved this study. This study number is #2013-77.
Tumor evaluation
The response status and the date of progression were determined according to the RECIST criteria version 1.1. The response rate (RR) was calculated as the percentage of patients who showed a complete (CR) or partial (PR) response among all patients, and the disease control rate (DCR) was calculated as the percentage of patients who showed a CR or PR and stable disease (SD) among all of the patients. The PFS was defined as the period from the first day of pembrolizumab treatment until the date of documentation of disease progression or death from any cause. The OS was defined as the period from the first day of pembrolizumab treatment to the date of death from any cause.
Statistical analyses
All of the statistical analyses were performed by a medical statistician (M Shimokawa) using the JMP software program (version 11.0). All of the statistical tests were two-sided, and P values of <0.05 were considered to indicate statistical significance. Categorical data were compared using Fisher’s exact test. The survival probability was estimated using the Kaplan-Meier method, and the difference in the probability of survival was analyzed using the Wilcoxon test. Multivariate analyses were performed using a proportional hazards regression model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. We performed a multivariate analysis for the survival using factors other than the “PD-L1 expression status” and “number of prior treatments”, as these are related to the CONUT score.
Results
Patients’ characteristics
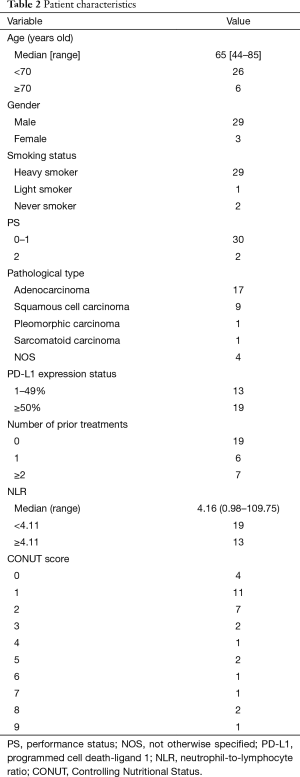
The patients’ baseline characteristics are summarized in Table 2. The median patient age was 65 years (range, 44–85 years), and 29 patients (90.6%) were male. Thirty (93.8%) had a smoking history, and 29 (90.6%) had a heavy smoking history (PYI ≥20). Two patients (6.3%) had an ECOG PS of 2. The pathological diagnoses of all patients were as follows: adenocarcinoma, n=17 (53.1%); squamous cell carcinoma, n=9 (28.1%); pleomorphic carcinoma, n=1 (3.1%); sarcomatoid carcinoma, n=1 (3.1%), and carcinoma not the otherwise specified (NOS), n=4 (12.5%). The NOS patients were classified into the non-sq population. Based on the evaluation of the PD-L1 expression, 13 patients (40.6%) were classified into the low expression group (1–49%), and the other 19 (59.4%) were classified into the high expression group (50–100%). Pembrolizumab was administered to 19 patients for the first treatment, 6 for the second treatment, and 7 for the third and subsequent treatments. All 19 patients who were treated with pembrolizumab as the first-line treatment had high PD-L1 expression. This means that the initial treatment group and the PD-L1 expression ≥50% group were the same population. The mean NLR was 4.16 (range, 0.98–109.75). Thirteen of the 32 (40.6%) patients were classified into the low NLR group (<4.11). The mean CONUT score was 2.59 (range, 0–9). Twenty-two of the 32 (68.8%) patients were classified into the low CONUT group (CONUT ≤2).
Full table
In 23 patients (71.9%), including 17 with adenocarcinoma, an analysis of the major genes associated with lung cancer was performed; a genetic analysis was not performed in the other 9 cases. In the 23 cases in which the analysis was performed, no targetable gene mutations were detected in EGFR, anaplastic lymphoma kinase (ALK), ROS1, or BRAF.
The relationship between CONUT and the other clinicopathological factors
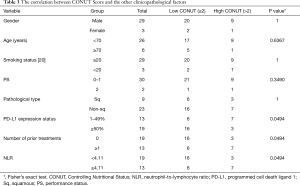
We evaluated the relationship between the CONUT score level and other clinicopathological factors, including the NLR using Fisher’s exact test (Table 3). There were no significant differences in the gender, age, smoking status, PFS, and pathological type of the high and low CONUT groups. On the other hand, the low CONUT group included a significantly higher percentage of patients who had PD-L1 expression status of 50% or more without prior treatment in comparison to the high CONUT group [16/22 (72.7%) vs. 3/10 (30.0%), respectively; P=0.0494]. In addition, the low CONUT group included a significantly higher percentage of patients with a low NLR in comparison to the high CONUT group [16/22 (72.7%) vs. 3/10 (30.0%), respectively; P=0.0494].
Full table
The response and survival
The clinical responses of all the patients were as follows: PR, n=14; SD, n=10; and progressive disease, n=8. Thus, the RR was 43.8% (14/32), and the DCR was 75.0% (24/32). The high NLR group had a significantly worse RR than the low NLR group (15.4% and 63.2%, P=0.0166) (Table 4). The high CONUT score group tended to have a worse DCR than the low CONUT score group (50.0% and 86.4%, P=0.0722). No other factors were associated with the RR or DCR.
Full table
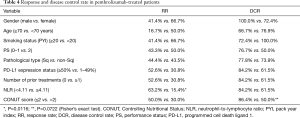
The PFS and OS of all the patients is shown in Figure 2. The 1-year PFS rate and median PFS were 46.1% and 10.3 months, respectively. The 1-year OS rate and median OS were 76.5% and not reached, respectively.
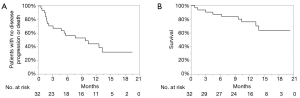
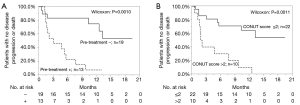
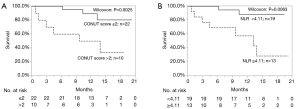
The univariate analyses revealed that the presence of prior treatment, and a high CONUT score were significantly associated with shorter PFS. A Kaplan-Meier survival analysis demonstrated that the 1-year PFS rate of patients with PD-L1 expression of ≥50% and no prior treatment was 76.3%, while that of the patients with prior treatment was 7.7% (P=0.0018, Wilcoxon test); the 1-year PFS rates of the low CONUT and high CONUT groups were 64.5% and 10.0%, respectively (P=0.0018, Wilcoxon test) (Table 5, Figure 3A,B). As shown in Table 6, the multivariate analysis showed that the CONUT score was found to be an independent predictor (HR, 0.33; 95% CI, 0.10–0.97; P=0.0435). Among patients without prior treatment, the high CONUT score group showed a significantly worse PFS than the low CONUT score group (1-year PFS rate: 33.3% vs. 84.0%; P=0.0024, Wilcoxon test) (Figure 4).

Full table

Full table
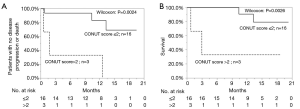
A univariate analysis of the factors associated with OS indicated that a high CONUT score and NLR were factors associated with worse survival. In a Kaplan-Meier survival analysis, the 1-year OS rates of the patients in the low and high CONUT groups were 89.3% and 50.0%, respectively (P=0.0025). The 1-year OS rate of patients with a low NLR was 88.4%, while that in patients with a high NLR was 57.7% (P=0.0063). A multivariate analysis showed that the CONUT score and NLR were the independent prognostic factors in NSLSC patients treated with pembrolizumab (HR, 0.15; 95% CI, 0.02–0.73, P=0.017; HR, 0.25; 95% CI, 0.05–0.98, P=0.048, respectively) (Tables 5,6, Figure 5). In the subgroup analysis of the no prior treatment group, the Kaplan-Meier survival analysis revealed that the high CONUT group had a significantly worse OS than the low CONUT group (1-year OS rate; 33.3% vs. 90.0%, P=0.0026) (Figure 4).
Discussion
The present study is the first to evaluate the relationship between ICI treatment and the nutritional status in NSCLC patients. We showed that OS and PFS of pembrolizumab-treated NSCLC patients in the high CONUT group (>2) were significantly poorer in comparison to the low CONUT group (≤2). In addition, the multivariate analysis revealed that the CONUT score was an independent predictor of the efficacy of pembrolizumab treatment and OS. The CONUT score could become a candidate early surrogate marker that can be used to select NSCLC who can be expected to benefit from pembrolizumab treatment.
The CONUT score is composed of 3 values, namely the levels of albumin and cholesterol and the total lymphocyte count in the peripheral blood. The serum albumin level reflects protein synthesis ability, the total cholesterol level reflects the lipid metabolism ability, and the total lymphocyte count reflects the immune function (11). The CONUT score was reported to be significantly associated with the Subjective Global Assessment (SGA), which is another nutritional index (kappa index, 0.488; P=0.034) (13). The SGA is simple, inexpensive and can be performed relatively quickly. However, it is a subjective evaluation that requires some skill and experience. In contrast, because the CONUT score is based solely on the results of the blood sample, the physician can easily perform and continuously evaluate the patient's nutritional state objectively during the course of treatment. Furthermore, as in the current study, the relevance of the score results to the clinical outcome can be retrospectively investigated. Thus, we conducted a retrospective analysis of the associations between the CONUT score and the outcomes of the patients in our study.
In our study, the proportion of patients with a lower CONUT score tended to be significantly higher among the patients who received pembrolizumab as an initial treatment in comparison to those who had a history of treatment (Table 3). A possible explanation is that the nutritional condition deteriorated due to previous treatment and that the PD-L1 expression level is related to the CONUT score. However, it was difficult to clarify this reason in this study, and further studies are planned to investigate the reason.
With regard to the significance of the CONUT score in cancer patients, several reports have described the prognostic impact of the CONUT score on the preoperative prognosis (15-18). Regarding NSCLC, some studies showed that the CONUT score was an independent prognostic factor for disease-free survival and OS in in patients with resected lung squamous cell carcinoma (19,24,25). Our previous report showed an association between the CONUT score and PFS and OS in MPM patients treated with chemotherapy (20). Daitoku et al. reported that patients with higher CONUT scores showed significantly shorter PFS (log-rank P<0.05) and OS (log-rank P<0.001) (21). Recently, the usefulness of the CONUT score for predicting the outcomes of adult T cell leukemia patients receiving mogamulizumab (a molecular targeted drugs) was reported. In that report, the median OS and non-relapse mortality (NRM) rate at 1 year among patients receiving allogeneic hematopoietic stem cell transplantation among patients with a CONUT score of 0–3 (n=10) were 1,685.5 days and 30%, respectively; in contrast, the values were 184.5 days and 100% in patients with a score of ≥4 (n=4) (P=0.017, OS; P=0.064, NRM). However, no studies have evaluated the relation between the CONUT score and the outcomes of NSCLC patients who receive chemotherapy or molecular targeted drugs such as TKIs. No studies have been performed on the usefulness of the CONUT score in predicting the outcomes of ICI treatment is patients with any type of carcinoma. In the present study, we showed that—for the first time—the OS and PFS of ICI-treated cancer patients with high CONUT scores were significantly poorer in comparison to those with low CONUT scores.
Pembrolizumab is a highly selective humanized monoclonal antibody against PD-1, which prevents PD-1 from engaging PD-L1 and PD-L2. The phase 1 KEYNOTE-001 and phase 3 KEYNOTE-010 studies showed that advanced NSCLC patients with a PD-L1 TPS of ≥50% were more likely to show a better response to pembrolizumab than those with a lower TPS (5,26,27). In the phase III trial (KEYNOTE-024), which compared the combination platinum therapy with pembrolizumab therapy for stage IV NSCLC with PD-L1 TPS of 50% or more, the primary endpoint, PFS, had an HR of 0.50 (10.3 vs. 6.0 months, 95% CI, 0.37–0.68, P<0.001) while the secondary endpoint, OS, had an HR 0.60 (95% CI, 0.41–0.89, P=0.005). It was shown that monotherapy with pembrolizumab significantly prolonged PFS and OS in comparison to treatment with conventional cytotoxic anticancer agent (7). In addition, another Phase III trial (KEYNOTE-042) investigated the OS after pembrolizumab monotherapy as first-line therapy in NSCLC patients with a PD-L1 TPS of ≥1% compared to standard chemotherapy. In the exploratory subgroup analysis of that study, the OS in the PD-L1 TPS of the 1–49% population seemed to be similar between the pembrolizumab and chemotherapy groups. The median OS was 13.4 months (95% CI, 10.7–18.2 months) in the pembrolizumab group and 12.1 months (95% CI, 11.0–14.0 months) in the chemotherapy group (8). However, treatment-related adverse events of grade ≥3 occurred in 113 (18%) of the 636 treated patients in the pembrolizumab group and in 252 (41%) of the 615 patients in the chemotherapy group in that study. The tumor cell expression of PD-L1 is thus the only established biomarker for pembrolizumab treatment in NSCLC patients, and pembrolizumab is currently recommended as the first-line therapy for NSCLC with PD-L1 TPS of ≥50% without any targetable gene mutations. Pembrolizumab is also a promising treatment option for NSCLC patients with TPS 1–49%. In the present study, the expression of PD-L1 was detected in all patients treated with pembrolizumab as first-line chemotherapy, whereas all patients treated with pembrolizumab as a secondary and subsequent chemotherapy were PD-L1-negative. The PFS period in the first-line treatment group was significantly better in comparison to the other groups (Figure 3A). Considering the CONUT score, even when the population was limited to the first-line treatment group, the patients with high CONUT values showed significantly poorer PFS and OS in comparison to those with low CONUT values (Figure 4).
Recently, in two randomized phase III clinical trials for squamous cell carcinoma and non-squamous cell carcinoma (KEYNOTE-407 and KEYNOTE-189, respectively), pembrolizumab was added to the conventional cytotoxic anticancer drug treatment in advanced lung cancer (28,29). In KEYNOTE-407, regardless of the PD-L1 expression, the addition of pembrolizumab to chemotherapy with carboplatin plus paclitaxel or nab-paclitaxel resulted in a significantly longer OS and PFS than chemotherapy alone for squamous NSCLC. In KEYNOTE-189, regardless of the PD-L1 expression, the addition of pembrolizumab to chemotherapy of pemetrexed and a platinum-based drug resulted in a significantly longer OS and PFS than chemotherapy alone for nonsquamous NSCLC without EGFR or ALK mutations. Based on these results, pembrolizumab plus cytotoxic anticancer drug treatment is expected to be an important option in the first-line treatment for metastatic recurrent NSCLC without any driver oncogenes. In the future, we would like to explore whether or not nutritional indicators, such as the CONUT score, are important in this treatment.
In recent years, inhibitors of the CTLA4-B7 pathway and the PD-1/PD-L1 pathway have come to play an important role as new cancer treatments. Research on tumor biomarkers associated with therapeutic effects has not only investigated the PD-L1 expression, it has also involved comprehensive gene expression analyses and cancer genome analyses, and genome-wide research is progressing. The level of tumor mutation burden (TMB) (30-32), the expression of MHC-II molecule (33), the CD8-expression of tumor-infiltrating lymphocytes (TILs) (34), neoantigens (35), and the lack of DNA mismatch repair system (34) have been proposed as candidate factors for predicting the effects of ICI therapy. In the peripheral blood, the expression of regulatory T cells/cancer antigen specific T cells (36) and specific inflammation and interferon-γ-related mRNA-based signatures (36), the NLR and the platelet-to-lymphocyte ratio have been proposed as the candidates (11,12,37). In this study, the NLR was associated with OS. However, the CONUT score and NLR were identified as independent factors in the multivariate analysis. It should be pointed out that studies on biomarkers are still in the experimental stage, and that none of the abovementioned markers has been used yet in actual clinical practice. The mutual relevance of these biomarkers is considered to be important, so we are planning future research.
The present study is associated with several limitations. First, it was a retrospective study that was conducted in a single-institution and the number of patients treated with pembrolizumab was relatively small. Furthermore, since the serum cholesterol level is not considered to be important in drug treatment for NSCLC, 17 patients who received pembrolizumab during the same period were excluded because their cholesterol levels were not measured. These 17 patients who were excluded in this analysis received pembrolizumab as second line or later treatment. So, this exclusion did not seem to effect on the association between the CONUT value and the prognosis of the patients who received pembrolizumab as a first-line regimen in the present study. Third, due to the retrospective nature of the study, the current study could not include factors that could impact the inflammation and nutritional statuses, such as medications and other medical conditions. Furthermore, some information regarding weight loss and the PS at the time of the diagnosis was unavailable. A prospective study should be performed to overcome these limitations.
In conclusion, the CONUT score may predict the therapeutic effects and prognosis of NSCLC patients treated with pembrolizumab. The present study suggests that in addition to the PD-L1 expression level, the CONUT score may play a major role in the selection of treatment for NSCLC patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. T Ohba reports personal fees from AstraZeneca, personal fees from Bristol-Myers Squibb, Chugai Pharmaceutical, and Nippon Boehringer Ingelheim. Dr. R Toyozawa reports personal fees from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly Japan, Kyowa Hakko Kirin, MSD, Nippon Boehringer Ingelheim, Nippon Kayaku, Ono Pharmaceutical, and Taiho Pharmaceutical. Dr. K Nosaki reports personal fees from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly Japan, Kyowa Hakko Kirin, Nippon Boehringer Ingelheim, Nippon Kayaku, Ono Pharmaceutical, Pfizer Japan, Taiho Pharmaceutical, and grants and personal fees from MSD, and Novartis Pharma. Dr. Miura reports personal fees from Ono Pharmaceutical. Dr. M Yamaguchi reports personal fees from Astellas Pharma, AstraZeneca, Chugai Pharmaceutical, Covidien Japan, Daiichi Sankyo, Eli Lilly Japan, Johnson & Johnson, Kyowa Hakko Kirin, Nippon Boehringer Ingelheim, Ono Pharmaceutical, and Taiho Pharmaceutical. Dr. K Taguchi reports personal fees from AstraZeneca, MSD, Ono Pharmaceutical, and Taiho Pharmaceutical. Dr. T Seto reports grants and personal fees from Astellas Pharma, AstraZeneca, Chugai Pharmaceutica, Eli Lilly Japan, Kissei Pharmaceutical, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Pfizer Japan, Takeda Pharmaceutical, and personal fees from Bristol-Myers Squibb, Kyowa Hakko Kirin, Nippon Kayaku, Ono Pharmaceutical, Roche Singapore, Taiho Pharmaceutical, Thermo Fisher Scientific, YakultHonsha, and grants from Bayer Yakuhin, Daiichi Sankyo, Eisai, LOXO Oncology, and Merck Serono. Dr. M Shimokawa reports consulting fee from Sysmex. Dr. M Takenoyama reports grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Covidien Japan, Eli Lilly Japan, Kyowa Hakko Kirin, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Ono Pharmaceutical, Taiho Pharmaceutical, and grants from Johnson & Johnson, Kaketsuken, and personal fees from Pfizer Japan. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The National Hospital Organization Kyushu Cancer Center Institutional Review Board approved this study (#2013-77).
References
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Melosky B, Chu Q, Juergens R, et al. Pointed Progress in Second-Line Advanced Non-Small-Cell Lung Cancer: The Rapidly Evolving Field of Checkpoint Inhibition. J Clin Oncol 2016;34:1676-88. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Martignoni ME, Kunze P, Friess H. Cancer cachexia. Mol Cancer 2003;2:36. [Crossref] [PubMed]
- Fearon KC, Voss AC, Hustead DS. Cancer Cachexia Study G. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006;83:1345-50. [Crossref] [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Sekine K, Kanda S, Goto Y, et al. Change in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer. Lung Cancer 2018;124:179-88. [Crossref] [PubMed]
- Ignacio de Ulíbarri J, Gonzalez-Madrono A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38-45. [PubMed]
- González-Madroño A, Mancha A, Rodriguez FJ, et al. Confirming the validity of the CONUT system for early detection and monitoring of clinical undernutrition: comparison with two logistic regression models developed using SGA as the gold standard. Nutr Hosp 2012;27:564-71. [PubMed]
- Ishihara H, Kondo T, Yoshida K, et al. Preoperative controlling nutritional status (CONUT) score as a novel predictive biomarker of survival in patients with localized urothelial carcinoma of the upper urinary tract treated with radical nephroureterectomy. Urol Oncol 2017;35:539.e9-539.e16. [Crossref] [PubMed]
- Harimoto N, Yoshizumi T, Inokuchi S, et al. Prognostic Significance of Preoperative Controlling Nutritional Status (CONUT) Score in Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma: A Multi-institutional Study. Ann Surg Oncol 2018;25:3316-23. [Crossref] [PubMed]
- Miyata T, Yamashita YI, Higashi T, et al. The Prognostic Impact of Controlling Nutritional Status (CONUT) in Intrahepatic Cholangiocarcinoma Following Curative Hepatectomy: A Retrospective Single Institution Study. World J Surg 2018;42:1085-91. [Crossref] [PubMed]
- Kuroda D, Sawayama H, Kurashige J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer 2018;21:204-12. [Crossref] [PubMed]
- Akamine T, Toyokawa G, Matsubara T, et al. Significance of the Preoperative CONUT Score in Predicting Postoperative Disease-free and Overall Survival in Patients with Lung Adenocarcinoma with Obstructive Lung Disease. Anticancer Res 2017;37:2735-42. [Crossref] [PubMed]
- Takamori S, Toyokawa G, Taguchi K, et al. The Controlling Nutritional Status Score Is a Significant Independent Predictor of Poor Prognosis in Patients With Malignant Pleural Mesothelioma. Clin Lung Cancer 2017;18:e303-13. [Crossref] [PubMed]
- Daitoku N, Miyamoto Y, Tokunaga R, et al. Controlling Nutritional Status (CONUT) Score Is a Prognostic Marker in Metastatic Colorectal Cancer Patients Receiving First-line Chemotherapy. Anticancer Res 2018;38:4883-8. [Crossref] [PubMed]
- Liu X, Zhang D, Lin E, et al. Preoperative controlling nutritional status (CONUT) score as a predictor of long-term outcome after curative resection followed by adjuvant chemotherapy in stage II-III gastric Cancer. BMC Cancer 2018;18:699. [Crossref] [PubMed]
- Teraoka S, Fujimoto D, Morimoto T, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thorac Oncol 2017;12:1798-805. [Crossref] [PubMed]
- Shoji F, Haratake N, Akamine T, et al. The Preoperative Controlling Nutritional Status Score Predicts Survival After Curative Surgery in Patients with Pathological Stage I Non-small Cell Lung Cancer. Anticancer Res 2017;37:741-7. [Crossref] [PubMed]
- Toyokawa G, Kozuma Y, Matsubara T, et al. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J Thorac Dis 2017;9:2942-51. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 2016;27:1291-8. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Johnson DB, Estrada MV, Salgado R, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun 2016;7:10582. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Gros A, Parkhurst MR, Tran E, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 2016;22:433-8. [Crossref] [PubMed]
- Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 2013;31:4311-8. [Crossref] [PubMed]
- Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1-7. [Crossref] [PubMed]


