Experimental studies in the bronchial circulation. Which is the ideal animal model?
Introduction
Pulmonary circulation carries deoxygenated blood away from the heart to the lungs, and returns oxygenated blood back to the heart. The separate system is known as the bronchial circulation supplies blood to the tissue of the larger airways of the lung. The first reference to some small arteries in the lungs was made by Galen (1). Many years later, in 1513, Leonardo Da Vinci, described the bronchial arterial system as subsidiary of pulmonary vessels (2). In 1721, Ruysch presented the first illustration and made a more detailed description of the bronchial arteries as a separate vascular network of the pulmonary vasculature with anastomoses between the two networks (3). In 1747, Albertus Haller gave a precise description of the origin, course and the variations of bronchial arteries in his masterpiece “Icorum Anatomicarum” (4).
Today, the importance of the bronchial arteries is recognized by the scientific community, especially in cases of massive hemoptysis and chemotherapy of lung cancer (5,6). Their revascularization seems to contribute to the reduction of postoperative complications in cases of heart-lung or single lung transplantation (7). In addition, the potential benefit from their embolism in cases of surgical treatment of thoracic or aneurysms of the descending aorta are still under investigation.
The ideal experimental studies should be made in models that show the greatest similarity to the human anatomy and physiology. Although, the most experimental studies in cardiopulmonary system take place mainly in the pigs. The anatomy of the porcine bronchial system has not yet been fully explored. Our aim is to explore which is the ideal experimental model for the bronchial circulation studies.
Methods
A thorough description and a comparative analysis of the anatomy of the bronchial system in human, pigs and other laboratory animals is presented in order to highlight the ideal animal model for experiments in the bronchial circulation.
The human bronchial circulation
Both left and right bronchial arteries originate from the aorta in 90%. More specifically in 80%, they originate between the upper border of the 5th and the lower border of the 6th thoracic vertebrae (T5-T6) with most of them arising from the level of the carina or slightly inferiorly (8,9). In the remaining cases, it has been described the origin of the bronchial arteries from all thoracic arterial structures, even from the coronary arteries (10). Cauldwel et al., based on a large human cadaveric study, classified the variations in the number of bronchial arteries in nine categories (11). In most cases, the right bronchial artery is one of the branches of the intercostobronchial trunk that originates from the aorta.
In their origin, the diameter of the bronchial arteries is about 1.5 mm, decreasing to 0.5-0.6 mm when enter the pulmonary hilum (12). In cases of chronic pulmonary diseases, especially when haemoptysis is present, hypertrophy and increase in the arterial diameter more than 1.5 mm have been observed (9,13).
In their path from the aorta to the hilum, the bronchial arteries give branches to adjacent organs and structures. Small parietal branches may supply muscles, vertebrae, ligaments and pleura. Visceral branches supply the esophagus, the lower third of the trachea and pericardium. Vascular branches supply the aorta, the pulmonary vasculature, the azygous vein and the vena cava. There are also branches to intrathoracic nerves, such as to the vagus nerve, the sympathetic plexus and their branches. In a few studies, branches to mediastinal lymph nodes and an anastomotic plexus with the coronary circulation are described (14-16).
The bronchial arteries enter the lung to the hilum. They form a communicating arc round the main bronchi from which the bronchial arterial divisions radiates along the major bronchi. They adhered closely to the bronchial wall and appeared to follow the same course to the level of the terminal bronchioles where finally anastomosed to the pulmonary vasculature (17). They bifurcated with the bronchi and give two to three divisions along each bronchus which tend to form an intercommunicating network in the fibrous coat of the bronchus. Smaller twigs penetrate the muscular layer and reach the bronchial mucosa forming a similar network in the submucosa. In this way, the bronchial arteries are responsible for the nourishment of the whole thickness of the bronchial wall till the level of terminal bronchioles. In the way down to the alveoli, the bronchial arterioles form an anastomotic network with pulmonary arterioles. These anastomotic branches, which named bronchopulmonary arteries, have been observed in both newborns and adults drain via the pulmonary veins into the left atrium (17-19). Tobin et al. described that there are two types of anastomoses, the short-narrow (length 1-2 mm, diameter 50-100 microns) and the long-wide (length 10-40 mm, diameter 300-400 microns) (20).
The short anastomotic vessels have spiral shape, achieving in this way the self-regulation of the flow to the pulmonary bronchial network and vice versa. Under normal conditions, these anastomoses are functionally closed but under pathologic conditions, such as in chronic thromboembolic pulmonary hypertension, they open and new anastomoses are formed (12,21,22).
Counterparts to the bronchial arteries are the bronchial veins. However, they only carry a small amount of the blood flow of the bronchial arteries while the remaining blood is returned to the heart via the pulmonary veins. The bronchial veins return blood from the larger bronchi and structures at the hilum of the lungs. The right side drains into the azygous vein, while the left side drains into the left superior intercostal vein or the accessory hemiazygous vein (17).
Comparative study of the bronchial circulation in laboratory animals
McLaughlin thoroughly described the pulmonary anatomy of 10 mammalian species and the human, classifying them into three groups according to their similarities in lung anatomy and physiology (23,24). In the first group belong the cow, the sheep and the pig. In the second group belong the dog, the cat and the monkey. Τhe rabbit, the rat and the guinea-pig could be considered a subcategory of the second group due to their minimum differences. Finally, in the third group belong the horse and the human.
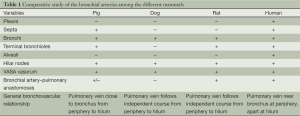
In the following presentation a representative from each group has been chosen based on the ease to perform experiments on this and the lower cost of its maintenance. Thus, the bronchial circulation of the pig, the dog and the rat is described along with this of birds as being the most commonly used laboratory animals. The differences among the bronchial circulation of the human, the pig, the rat and the dog are presented in the Table 1.
Full table
Bronchial circulation of the pig
Calka et al., Gade et al., and more recently Lorentziadis et al., demonstrated the most detailed descriptions of the anatomy of the porcine bronchial tree (25-27). The bronchial artery is the continuation of the bronchoesophageal artery which almost always originates from the aorta as a single vessel (28).
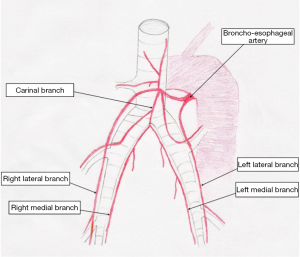
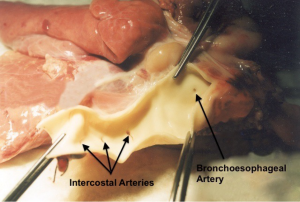
In 91-100% has a single origin from the aorta (Figure 1). In about 85% originates from the medial or anteromedial aspect of the descending aorta just distal to the ductus arteriosus ligament but cranial to the azygos vein crossing the aorta. In about 15% the origin is more mediodorsal or just cranial to the ductus arteriosus ligament. The orifice is normally 1-2 mm wide and approximately 3 cm from the slightly larger orifices of the intercostal arteries (25). The bronchoesophageal artery soon divides into 2-3 branches towards the lung hilum and the esophagus, while three different patterns of branching in the way to the main bronchi have been described (22) (Figure 2).
In details, the bronchoesophageal artery is divided to a carinal branch, a left lateral branch and a smaller branch for the cranial lobe. The carinal branch crosses the carina ventrally and ramified further into a right lateral, a right medial and a left medial branch. The right lateral and right medial branches provide blood supply to the right main bronchus while the left lateral and left medial branches provide blood supply to the left main bronchus. According to the pattern described, each main bronchus of the pig is accompanied by two major bronchial artery blanches named in accordance with their anatomical localization (26). Instead of this, the segmental bronchi are accompanied by a single bronchial branch, which finally disappeared 1-2 cm before the edge of the lung. All branches of the bronchial artery are in close proximity to the adjacent bronchi.
Communications between the bronchial branches and other structures are demonstrated in different levels. In the way to the hilum, small twigs develop an anastomotic plexus with most of the mediastinal structures including the esophagus, the pericardium, lymph nodes and vasa vasorum to the pulmonary artery (23,29). The existence of an anastomotic plexus with the pulmonary artery is still under question. Gade et al. demonstrated that broncho-pulmonary shunts must exist but it is likely to occur via the lung capillaries (26), though other investigations failed to demonstrated bronchial to pulmonary artery communications probably due to limitations in methodology. Other studies suggested that the communication between the two systems is via small pulmonary arterioles (23).
Of main importance is the anastomotic plexus with the esophagus along its entire intrathoracic length (30) while the existence of an anastomotic network between the bronchial branches and the coronary vessels of the heart has been described (31,32).
Bronchial circulation of the dog
There is significant anatomical variation of the origin of the bronchial arteries in dogs (33). The bronchial artery could be branch of the right 5th, 6th or 7th intercostal artery. These intercostal arteries always arise from the thoracic aorta. In the majority of dogs the parent trunk is the bronchoesophageal artery, which arises from the right fifth intercostal artery close to its origin from the aorta (34). The course followed by the bronchial artery is also subject to considerable variation. In the majority of dogs the bronchoesophageal artery crosses the left side of the esophagus and contributes an esophageal branch before entering the hilum of the lung (34). In addition, small bronchial vessels that supply the hilum of the lung and arise from the pericardiophrenic or internal thoracic arteries are described. At the level of the respiratory bronchiole the bronchial artery terminates in a capillary bed that is continuous with that of the pulmonary artery (35).
True bronchial veins are found only at the hilum of the lung. They empty into the azygos vein or the intercostal vein at the level of the seventh thoracic vertebra (34).
Bronchial circulation of the rat
There are two bronchial arteries, the right and the left, which originate either from the subclavian arteries or from their primary branches (36). Each of the bronchial arteries has a long caudal course through the mediastinum, supplying several thoracic structures other than the bronchi. The left bronchial artery always originates from the internal thoracic artery. The right bronchial artery had a variable origin: the costocervical trunk, the right subclavian artery, or the internal thoracic artery (37). After emerging from the internal thoracic artery, the left bronchial artery run caudally on the ventral surface of the aortic arch, where it gives off branches to the thymus, the trachea and the left recurrent laryngeal nerve. At the dorsal level of the left bronchus, the left bronchial artery originates esophageal and bronchial arteries. One or two branches run down along the main bronchus. The right bronchial artery gives off branches to the trachea, right cranial vena cava, and phrenic nerve. At the level of the carina, it originates 1-3 branches to the cranial bronchus and numerous transverse branches to the esophagus (38). Intrapulmonary branches of bronchial arteries form a peribronchial plexus made up of anastomosing arterioles giving off branches that passed through the muscular layer to form a second layer under the epithelium. Finally, precapillary anastomoses between bronchial arteries and pulmonary vessels exist resembling what occurs in humans (39).
Bronchial circulation of birds
General studies of the avian arterial system (40,41) have indicated that bronchial arteries arise from the oesophagotracheobronchial branch of each common carotid artery in many species of birds. Each oesophagotracheobronchial artery passes caudally and forms more or less symmetrically, three or four branches supplying the caudal end of the trachea, the syrinx, and the oesophagus. It also gives rise to several small bronchial arteries which supply the extrapulmonary part of the primary bronchus. The largest and most caudal of these bronchial arteries passes caudally along the dorsal wall of its primary bronchus and enters the hilus of the lung. On reaching the opening of the first medioventral secondary bronchus it divides into two terminal branches which continue caudally on either side of the openings of the medioventral secondary bronchi, forming a network of small anastomosing branches. In the goose, duck, and turkey several small bronchial arteries supplied the whole length of each primary bronchus, including the orifices of the secondary bronchi. In the guinea-fowl and quail similar bronchial arteries supplied only the extrapulmonary part of the primary bronchus. In the pigeon a single true bronchial artery supplied the extrapulmonary part of each primary bronchus. There were no branches to the exchange tissue in any species. In all species the bronchial veins of the extra-pulmonary part of the primary bronchus drained via esophageal veins, whereas those of the intrapulmonary part emptied into branches of the pulmonary vein (40,41).
Results and discussion
The ideal experimental model should demonstrate the maximum similarity to the humans, in both anatomy and physiology. Lungs of all mammals share a common general structure consisting of a branching system of airways terminating in thin-walled alveolar spaces where gas exchange occurs but there are variations among species (23). Pigs, sheep and cows having “type I” lungs characterized by extremely well developed lobulation of lung parenchyma, a thick visceral pleura and absence of alveoli. The pulmonary artery supplies the distal portion of the airways. The bronchial artery supplies the hilar lymph nodes, pulmonary artery and vein, bronchi and terminal airways, but it also supplies the interlobular septae and pleura.
Dogs, cats and monkeys having a “type II” lungs characterized by the absence of secondary lobules within lobes, ill-defined intraparenchymal connective tissue support and a thin membraneous pleura. The pulmonary artery supplies the distal portion of the respiratory bronchiole, the alveolar duct, alveoli and pleura. The bronchial artery, except for a few short branches near the hilum contributes none of the pleural supply, but does supply the hilar lymph nodes, pulmonary artery and vein, bronchi and bronchioles and terminates in a common capillary bed with the pulmonary artery at the respiratory bronchioles. Rats, rabbits and guinea-pigs differ slightly as they have well-developed precapillary anastomoses.
human and horse having “type III” lungs characterized by partially developed secondary lobules with well-defined but haphazardly arranged interlobular septae and a thick vascular pleura. The pulmonary artery supplies the alveoli with only occasional anastomoses with bronchial artery at the terminal bronchial level. The bronchial artery supplies the hilar lymph nodes, pulmonary artery and vein bronchi and terminal airways, but it also supplies the interlobular septae and pleura. The bronchial artery contributes blood directly to the alveolar capillary network by the terminal bronchioles, interlobular septae and pleural network in areas lying close to the pleura.
Even if the horse has the same bronchial and pulmonary anatomy to humans its experimental use is extremely difficult because of its size, maintenance costs and animal testing regulations and laws.
In dogs, there is significant anatomical variation of the origin of the bronchial arteries and the course followed by the bronchial artery is also subject to considerable variation (33,34). There is also difficulty in isolation of the true bronchial arteries from their widespread anastomoses with the mediastinal and pericardial vessels (33).
As previously described the right bronchial artery of the rat concealed by the right vena cava and the phrenic nerve rendered its identification by dissection difficult (37). Additionally in rat, bronchial artery coming from the aorta is a rare event and correspond to a supernumerary artery, thus it is clear that the origin of bronchial arteries is clearly different in the rat and humans. On the other hand the presence of extrapulmonary branches of bronchial artery especially to the pericardium and myocardium is an important fact that suggests the existence of anastomoses between bronchial artery and the coronary circulation in the rat. This vascular arrangement may work as a collateral source of blood that reaches the myocardium (42). Thus, the rat may offer a model of coronary-bronchial arterial anastomoses that may be of interest to study the role of these anastomoses in heart and lung disorders in humans.
In birds, the bronchial arteries come from the bronchoesophageal artery, a branch of the common carotid artery and they express characteristic symmetry to their origin and their distribution (41). This pattern is clearly different to the human analog.
The pig and the sheep have the advantage that the broncho-oesophageal artery usually originates from the aorta as a single vessel (25,26,43). The relative constancy of a single arterial trunk makes the recognition and dissection of the artery easy to perform. In this way it is also easy to perform physiological and rheometric studies requiring catheterization of the vessel (32). The extrapulmonary topographic anatomy of bronchial arteries in pigs exhibits similarities to that of humans. The principal bronchi of the pig are each accompanied by two major bronchial artery branches. Two bronchial arteries for each lung is also a common finding in human. Furthermore, the growth of the heart and cardiovascular system from birth to 4 months of age is analogous to the growth of the same system in humans into the mid-teens (26).
Conclusions
The above mentioned, suggest that the pig and the sheep are anatomically and physiologically suited for experimental studies on the bronchial circulation. The bronchial artery of the pig is similar to this of human concerning the origin, the course, the branches and the blood supply. The suitable bronchial anatomy and physiology along with the undeniable usefulness of the pig in experimental research and the low maintenance cost make the pig the ideal model for experiments in bronchial circulation.
Acknowledgements
The authors would like to express their gratitude to Mrs Alexia Agrogianni for her invaluable help in the creation of the figure of this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Galen C. Tracta Frobenianae. Book 6. Basel: 1562.
- Cudkowicz L. Leonardo de Vinci and the bronchial circulation. Br J Tuberc Dis Chest 1953;47:23-5. [PubMed]
- Ruysch F. eds. Opera omnia anatomico-medico-chirurgica. Vol I: Dilucidatio Valvularum, chap. 4, obs 15, 19-22, fig. 9. Amstelodami: Janssonio-Waesbergios, 1721.
- Haller Albertus. eds. Icorum anatomicarum quibus praecipuae partes corporis humani. Gottingen: 1747:35-7.
- Osaki T, Hanagiri T, Nakanishi R, et al. Bronchial arterial infusion is an effective therapeutic modality for centrally located early-stage lung cancer: results of a pilot study. Chest 1999;115:1424-8. [PubMed]
- Yoon W, Kim JK, Kim YH, et al. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395-409. [PubMed]
- Kamler M, Nowak K, Bock M, et al. Bronchial artery revascularization restores peribronchial tissue oxygenation after lung transplantation. J Heart Lung Transplant 2004;23:763-6. [PubMed]
- Moberg A. Anatomical and functional aspects of extracardial anastomoses to the coronary arteries. Pathol Microbiol (Basel) 1967;30:689-94. [PubMed]
- Remy-Jardin M, Bouaziz N, Dumont P, et al. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004;233:741-9. [PubMed]
- Liebow AA. Patterns of origin and distribution of the major bronchial arteries in man. Am J Anat 1965;117:19-32. [PubMed]
- Cauldwell EW, Siekert RG, et al. The bronchial arteries; an anatomic study of 150 human cadavers. Surg Gynecol Obstet 1948;86:395-412. [PubMed]
- Pump KK. Distribution of bronchial arteries in the human lung. Chest 1972;62:447-451. [PubMed]
- Petelenz T. Non-coronary anastomoses of bronchial arteries. Cardiologia 1967;50:43-55. [PubMed]
- Björk L. Angiographic demonstration of extracardial anastomoses to the coronary arteries. Radiology 1966;87:274-7. [PubMed]
- Moberg A. Anatomical and functional aspects of extracardial anastomoses to the coronary arteries. Pathol Microbiol (Basel) 1967;30:689-94. [PubMed]
- Dupont P, Riquet M. The bronchial arteries. A review of their anatomy and their anastomoses with the coronary arteries. Surg Radiol Anat 1991;13:69-71. [PubMed]
- Deffebach ME, Charan NB, Lakshminarayan S, et al. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987;135:463-81. [PubMed]
- Wagenvoort CA, Wagenvoort N. Arterial anastomoses, bronchopulmonary arteries, and pulmobronchial arteries in perinatal lungs. Lab Invest 1967;16:13-24. [PubMed]
- Pump KK. The bronchial arteries and their anastomoses in the human lung. Dis Chest 1963;43:245-55. [PubMed]
- Tobin CE. The bronchial arteries and their connections with other vessels in the human lung. Surg Gynecol Obstet 1952;95:741-50. [PubMed]
- Viamonte M Jr, Viamonte M, Camacho M, et al. Corrosion cast studies of the bronchial arteries. Surg Radiol Anat 1989;11:215-9. [PubMed]
- Baile EM. The anatomy and physiology of the bronchial circulation. J Aerosol Med 1996;9:1-6. [PubMed]
- McLaughlin RF, Tyler WS, Canada RO. A study of the subgross pulmonary anatomy in various mammals. Am J Anat 1961;108:149-65.
- Magno M. Comparative anatomy of the tracheobronchial circulation. Eur Respir J Suppl 1990;12:557s-62s; discussion 562s-3s.
- Calka W. Extrapulmonary course of the bronchial and bronchoesophageal arteries in the domestic pig. Folia Morphol (Warsz) 1975;34:59-67. [PubMed]
- Gade J, Norgaard MA, Andersen CB, et al. The porcine bronchial artery: surgical and angiographic anatomy. J Anat 1999;194:241-7. [PubMed]
- Lorentziadis M, Chamogeorgakis T, Toumpoulis IK, et al. Topographic anatomy of bronchial arteries in the pig: a corrosion cast study. J Anat 2005;207:427-32. [PubMed]
- Nathan H, Orda R, Barkay M. The right bronchial artery. Anatomical considerations and surgical approach. Thorax 1970;25:328-33. [PubMed]
- Remy J, Deschildre F, Artaud D, et al. Bronchial arteries in the pig before and after permanent pulmonary artery occlusion. Invest Radiol 1997;32:218-24. [PubMed]
- Gade J, Nørgaard MA, Andersen CB, et al. The porcine bronchial artery. Anastomoses with oesophageal, coronary and intercostal arteries. J Anat 1999;195:65-73. [PubMed]
- White FC, Carroll SM, Magnet A, et al. Coronary collateral development in swine after coronary artery occlusion. Circ Res 1992;71:1490-500. [PubMed]
- Kotoulas C, Karnabatidis D, Kalogeropoulou C, et al. Anastomoses between bronchial and coronary circulation in a porcine model: computed tomographic and angiographic demonstration. Hellenic J Cardiol 2006;47:206-10. [PubMed]
- Collier PS. Bronchial arteries of the dog-a pharmaco-anatomical study. J Anat 1979;128:887-8. [PubMed]
- Evans HE, De Lahundra A. eds. Miller’s anatomy of the dog. Fourth ed. Philadelphia: Elsevier Saunders, 2013:359.
- Notkovich H. The anatomy of the bronchial arteries of the dog. J Thorac Surg 1957;33:242-53. [PubMed]
- Hebel R, Strobberg MW. Circulatory system. In: Hebel R, Strobberg MW. eds. Anatomy of the laboratory rat. Baltimore: Williams & Wilkins, 1976:91.
- Ferreira PG, Silva AC, Aguas AP, et al. Detailed arrangement of the bronchial arteries in the Wistar rat: Astudy using vascular injection and scanning electron microscopy. Eur J Anat 2001;5:67-76.
- Deffebach M, Widdicombe J. The bronchial circulation. In: Crystal RG, West JB, Barnes PJ, et al. eds. The Lung: Scientific Foundations. New York: Raven Press, 1991:741-57.
- Peão MN, Aguas AP, de Sá CM, et al. Neoformation of blood vessels in association with rat lung fibrosis induced by bleomycin. Anat Rec 1994;238:57-67. [PubMed]
- Glenny FH. Modifications of pattern in the aortic arch system of birds and their phylogenetic significance. Proc U S Natl Mus 1955;104:525-621.
- Abdalla MA, King AS. The avian bronchial arteries: species variations. J Anat 1977;123:697-704. [PubMed]
- Moberg A. Anastomoses between extracardiac vessels and coronary arteries. 3. Microangiographic appearance. Acta Radiol Diagn (Stockh) 1968;7:33-47. [PubMed]
- Nazari S, Prati U, Berti A, et al. Successful bronchial revascularization in experimental single lung transplantation. Eur J Cardiothorac Surg 1990;4:561-6; discussion 567. [PubMed]