
Use of a modified endotracheal tube for self-expandable metallic Y-shaped airway stent deployment without rigid bronchoscope or fluoroscopic guidance
Introduction
Airway stents provide effective palliation for malignant and benign tracheobronchial stenosis and tracheoesophageal fistula.(1,2) A Y-shaped airway stent consists of a proximal limb situated at the trachea and two distal limbs extending into the two main bronchi with the bifurcation seated at the carina, suitable for lesions involving distal trachea and proximal main bronchi.(3,4) The Y-shaped stents used in our respiratory center is a self-expandable stent of nitinol mesh with an optional silicone covering and a dedicated loading device (the delivery catheter, 8 mm in diameter and 600 mm in length) (Micro-Tech, Nanjing, China), which is also widely utilized around the world (5-8).
Unlike straight stents, for which the delivery catheters are slim enough to pass through the nasal cavity and therefore could glide smoothly along the posterior pharyngeal wall into the glottis, Y-shaped stents require delivery catheters of larger caliber and thus need to be placed perorally, during which process a curvature around the epiglottis hinders the passage into the trachea (Figure 1), making it difficult and risky to place Y-shaped stents under flexible bronchoscopy, so generally the placement of such airway stents is performed under rigid bronchoscopy (7-9) and/or with the assistance of fluoroscopy (5,6,8,10,11), both of which are not yet widely accessible in institutions of developing countries and regions where such stents are all the more needed despite the lack of facilities.
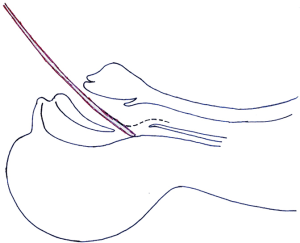
To overcome these difficulties, we have recently developed a new technique for the deployment of self-expandable metallic Y-shaped airway stents (SEMYS) under flexible bronchoscope using a slightly modified 7.5 or 8 internal diameter endotracheal tube to bypass the difficult turn of the pharynx, without need of rigid bronchoscopy or fluoroscopy. With properly selected patients, the procedure as described herein is safe, effective and simple to perform.
Methods
Patients
All patients with a tracheobronchial stenosis or fistula in the carinal regions presenting to the Department of Respiratory and Critical Care Medicine of the First Affiliated Hospital of Chongqing Medical University from September 2016 to December 2018 were screened for inclusion. Patients with severe contraindications of bronchoscopic examinations were excluded. Tracheoesophageal stenosis or fistula were diagnosed from patients' history, chest radiology and bronchoscopic findings. During the study period, all eligible patients with informed consent underwent stent deployment with the modified technique, but rigid bronchoscopy was available as a backup in case of deployment difficulties and emergencies.
Informed consent was acquired before the procedure, and the retrieval of patient data was approved by the Institutional Review Board of the hospital approval number: 2017-057.
Procedure
Setting and sedation
The procedure was carried out by a team of experienced bronchoscopists and nurses in a regular bronchoscopic suite under moderate sedation with intravenous propofol (1.5–2.5 mg/kg) and remifentanil (1–2 µg/kg). Spontaneous breathing was maintained and high-flow oxygen was delivered continuously through a nasal catheter.
Modification of an endotracheal tube into the deployment-assisting device
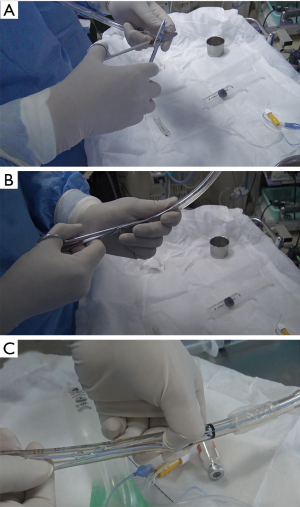
The deployment-assisting device, made of a 7.5 or 8 internal diameter endotracheal tube (Covidien Inc., Mexico) and specially designed to guide the delivery catheter through the S-shaped curvature of the glottis is crucial for the procedure. Accessories and the narrow proximal end of the tube is removed, and then the tube is cut open lengthwise (Figure 2). This endotracheal tube serves as a means to secure the airway and a deployment-assisting device simultaneously. The tube could provide adequate space for the delivery catheter to pass through, guiding it into the airway, and it is easy to remove through the lengthwise cut once the delivery catheter has been placed.
Stent deployment
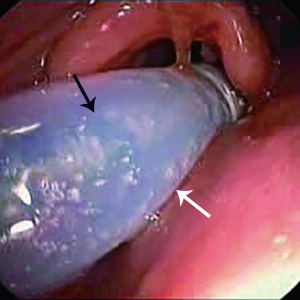
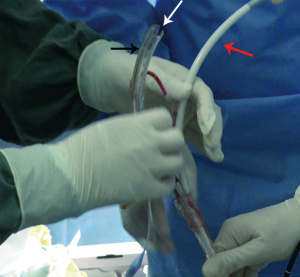
The deployment-assisting device, i.e., the modified endotracheal tube, was lubricated inside and out with liquid paraffin and intubated into the trachea with the guidance of flexible bronchoscopy (BF260, Olympus, Japan). After a guide wire (G-240-3545S, Olympus, Japan) was inserted through the bronchoscope into the target main bronchus where the longer distal limb of the SEMYS would be placed, the bronchoscope was withdrawn and reinserted through the nasal cavity to the glottis level, and then the delivery catheter was introduced perorally along the guide wire and through the deployment-assisting device, into the trachea, passing through the glottis curvature effortlessly (Figure 3). When the delivery catheter was securely placed in the trachea, the deployment-assisting device was retracted and removed from the delivery catheter through the slit (Figure 4). The flexible bronchoscope was then advanced into the trachea beside the delivery catheter to monitor the further course of stent deployment. The delivery catheter continued to advance in the trachea until its distal tip approached the carina, where the first lock of the launching gear was unlatched to release the two distal limbs, the longer of which was inserted along the guide wire into one of the main bronchi and the shorter into the other main bronchus (Figure 5), the bifurcation on the carina. Upon confirming with the bronchoscope that the two distal limbs are in place in the designated bronchi and, notably, not in the fistula, the second lock was unlatched, releasing the tracheal limb. After bronchoscopic inspection, the deployment procedure was completed, with the SEMYS firmly in place and the fistula effectively blocked (Figure 6). If the spontaneous expansion of the SEMYS was not complete, balloon catheters may be used to assist its expansion.
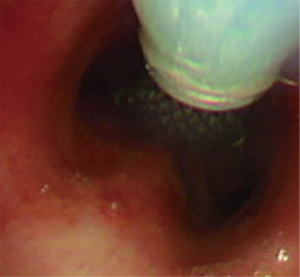
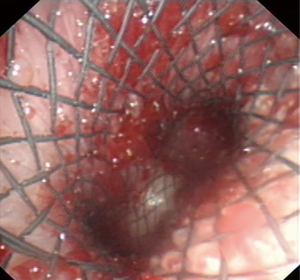
Flexible bronchoscopy was repeated one week after the procedure in all patients with successful placement of SEMYS.
Precaution against mid-deployment hypoxia
Because the procedures were carried out with spontaneous breathing, precaution should be taken to secure the process against hypoxia during the procedure, which involves the whole perioperative period. Patient selection is the important first step, and proficiency of the bronchoscopists is vital to ensure hemodynamic stability during the procedure. Emergency supplies including laryngeal masks and endotracheal tubes should be prepared readily available. Preoxygenation and denitrogenation should be achieved prior to the procedure, and depth of anesthesia should be handled with care. The patients were then placed in supine position with the neck extended to keep the airway open, and high flow oxygen was given through a nasal catheter. Continuous monitoring of oxygen saturation (SpO2) during the procedure assured immediate response to hypoxia. Once it occurred, the apparatus in the airway was removed at once and continuous positive airway pressure ventilation was instantly applied until restoration of normal SpO2 level, and then the procedure was resumed.
Results
Twenty patients were enrolled. Pathologies included pulmonary/esophageal cancer with tracheal/bronchial infiltration or fistulae (n=19; 95.0%) and tracheobronchial tuberculosis (n=1; 5.0%). The mean age of the study population was 58 years.
The stents used were specifically customized to fit the patients’ airway which was measured based on chest CT scan. Specifically, the diameter of the stents for patients with fistulas were 10% or 2 mm larger than the measured diameter of the airway as described in the Chinese expert consensus (12) while the diameter of stents for other patients were the same as the measured diameter of the airway. The diameter of the stents used in the study were 14–20 mm for the proximal limb and 10–12 mm for the distal limbs.
Successful deployment of SEMYS with the above described technique was carried out in 17 out of the 20 patients (85.0%). The duration from intubation to the end of the procedure was in the range of 18–120 min, and the deployment procedure from the insertion of the guide wire until the stent is fully released takes 5 to 25 min (Figure 7).
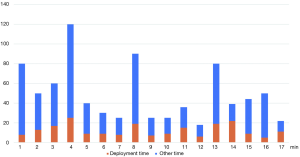
No cases of stent misplacement were encountered. Throughout the stent deployment process, SpO2 was maintained above 90% in all but one patient. The patient experienced a temporary drop in SpO2 to 65% during expansion of the distal stent because of the obstruction in the right main bronchus, but it was restored soon after removal of the obstruction. There was no hemodynamic compromise in all the procedures, neither was there any injury to the pharynx, vocal cords and the tracheobronchial tree. None of the patients complained of any major discomfort during and after the procedure.
Three patients required a conversion to rigid bronchoscopy. One of them had a short, thick neck and therefore a narrow pharyngeal cavity, making it extremely difficult for the delivery catheter to bend over the guide wire and advance, another experienced heavy bleeding during removal of his previous stent and was considered unfit for immediate SEMYS placement, and in another patient the airway was heavily scarred from tuberculosis infection and therefore especially prone to bleeding.
Follow-up bronchoscopic inspection one week after the procedure revealed the stents in place and no signs of migration. All the patients stated that their clinical symptoms had significantly improved post-operatively.
Discussion
SEMYS are of great use in patients with central airway stenosis and fistula, both malignant and benign. The stent sitting at the carina provides support for both proximal and distal airway around it. Currently the predominant method of SEMYS deployment requires rigid bronchoscopy and fluoroscopy for a stable operational passage and effective guidance (1,7,13,14). Such apparatuses are not widely accessible, and it is not practical to always transfer patients to more advanced centers in other regions or countries when they need SEMYS deployment.
While rigid bronchoscopy and fluoroscopy are definitely necessary for patients who are not stable enough or have specific airway issues in the process of SEMYS deployment, there are patients who are suitable to undergo SEMYS deployment without rigid bronchoscopy and fluoroscopy if the procedure is handled with the right technique and carefulness. In addition, given the high standard of technical proficiency, operation facility and multidisciplinary teams needed for rigid bronchoscopy and fluoroscopy, even in the institutions equipped with such facilities, SEMYS deployment with flexible bronchoscopy can also be a tempting alternative considering convenience and cost-effectiveness.
There are previous reports of SEMYS deployment with flexible bronchoscopy with the use of a spatula or laryngoscope, but our trial experience with these were not satisfactory. We have reported another simplified method for SEMYS deployment without the use of rigid bronchoscopy or fluoroscopy, in which the bronchoscopist uses the index finger to lift the tip of the delivery catheter and guide it into the trachea. Ten SEMYS were placed successfully with this technique, but this technique is difficult even for bronchoscopists with a high level of expertise, and a jet ventilator was needed. However, the deployment-assisting device described herein overcomes the shortness of the previous simplified technique.
To our knowledge, this is the first report of the placement of self-expanding Y-shaped stents with no need for rigid bronchoscopy and fluoroscopic guidance. The modification of the endotracheal tube into a deployment-assisting device is simple and practical, which adequately permits passage for the delivery catheter (7.5 or 8 mm in diameter) and does not take up so much space in the glottis as to result in airway obstruction. The slit of the endotracheal tube allows for smooth passage of the delivery catheter as well as convenient removal of the deployment-assisting device. Without the use of rigid bronchoscopy and fluoroscopy, the anesthesia procedure and accompanying apparatuses for the presented method are also simplified to a large extent.
The procedures were well tolerated under moderate sedation and spontaneous breathing, none of the patients experienced coughing or fidgety, and the procedures were all completed without persistent hypoxia, respiratory depression or hemodynamic instability, which is remarkable considering the infliction of moderate to severe airway stenosis or fistula, which are not easy to manage even with rigid bronchoscopy and fluoroscopy.
The few patients for whom the SEMYS were not placed successfully indicates the importance of proper selection of candidates for the procedure. From our experience, main counter indications for the flexible method include: (I) stenosis of the trachea, minimal diameter <11 mm; (II) swollen pharynx, especially when accompanied with a short neck; (III) moderate to severe bleeding according to the BTS guideline (15).
With the modified endotracheal tube and the dedicated delivery catheter, deployment of the SEMYS into its indicated position could be accomplished with ease. None of the patients experienced stent misplacement requiring instant removal of the stents, and slight adjustments could be made with forceps and balloon catheters. When necessary, removal of the stents would be facile since the laryngeal mask and even rigid bronchoscopy (if available) were readily available.
It is important that the aim of the simplified procedure is not to completely eliminate the use of rigid bronchoscopy and fluoroscopy, but to serve as a flexible alternative so that selected patients meeting certain criteria may benefit from the convenience and low cost of the flexible bronchoscopy. The procedure is of the highest level in the Chinese system of credentialing and privileging of interventional procedures, which is involved with the highest level of risk, complexity and difficulty, and the bronchoscopist need to be properly trained, proficient and experienced in bronchoscopic stent deployment to perform the procedure in order to ensure safety of the process and quality of care.
This presented method, using an endotracheal tube to secure the airway and guide the delivery device, provides a simple and practical approach to the deployment of SEMYS without rigid bronchoscopy and fluoroscopy, and the equipment accessibility makes it currently the most facile, efficient and cost-effective method for the procedure. However, the present study is limited by its single-center nature and small sample size, and it needs to be further validated with a prospective randomized control trial.
Another issue is the regulatory limitations of the off-label use and the modification of the endotracheal tubes, which could result in severe legal consequences. In our study, details of the modification and the related risks were carefully explained to every patient and informed consent regarding use of the currently unapproved medical device was obtained before each procedure, but the ultimate solution would be the cooperation with industrial partners.
In conclusion, while deployment under rigid bronchoscopy remains the recommended modality of choice for greater safety concerns, this presented method, given technical proficiency of the bronchoscopist, cautious patient selection and adequate vital sign monitor, is ideal for institutions without the privileges of rigid bronchoscopy and fluoroscopy.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was acquired before the procedure, and the retrieval of patient data was approved by the Institutional Review Board of the Hospital (2017-057).
References
- Guibert N, Mazieres J, Marquette CH, et al. Integration of interventional bronchoscopy in the management of lung cancer. Eur Respir Rev 2015;24:378-91. [Crossref] [PubMed]
- Sahebazamani M, Rubio E, Boyd M. Airway gastric fistula after esophagectomy for esophageal cancer. Ann Thorac Surg 2012;93:988-90. [Crossref] [PubMed]
- Nam HS, Um SW, Koh WJ, et al. Clinical application of the Natural Y stent in the management of benign carinal stenosis. Ann Thorac Surg 2009;88:432-9. [Crossref] [PubMed]
- Li TF, Duan XH, Han XW, et al. Application of combined-type Y-shaped covered metallic stents for the treatment of gastrotracheal fistulas and gastrobronchial fistulas. J Thorac Cardiovasc Surg 2016;152:557-63. [Crossref] [PubMed]
- Yang RM, Han XW, Wu G, et al. Implantation of a self-expandable metallic inverted Y-stent to treat tracheobronchial stenosis in the carinal region: initial clinical experience. Clin Radiol 2007;62:1223-8. [Crossref] [PubMed]
- Han XW, Wu G, Li YD, et al. Overcoming the delivery limitation: results of an approach to implanting an integrated self-expanding Y-shaped metallic stent in the carina. J Vasc Interv Radiol 2008;19:742-7. [Crossref] [PubMed]
- Özdemir C, Sökücü SN, Karasulu L, et al. Placement of self-expandable bifurcated metallic stents without use of fluoroscopic and guidewire guidance to palliate central airway lesions. Multidiscip Respir Med 2016;11:15. [Crossref] [PubMed]
- Madan K, Dhooria S, Sehgal IS, et al. A Multicenter Experience With the Placement of Self-Expanding Metallic Tracheobronchial Y Stents. J Bronchology Interv Pulmonol 2016;23:29-38. [Crossref] [PubMed]
- Monnier Y, Chollet-Rivier M, Gonzalez M, et al. Use of combined suspension laryngoscopy and jet ventilation for Y-shaped airway stents delivery. Ann Thorac Surg 2014;97:2208-10. [Crossref] [PubMed]
- Qiao Y, Fu YF, Cheng L, et al. Placement of integrated self-expanding Y-shaped airway stent in management of carinal stenosis. Radiol Med 2016;121:744-50. [Crossref] [PubMed]
- Wei N, Fu YF, Zhang K, et al. Ventilation catheter-assisted airway stenting under local anaesthesia for patients with airway stenosis: initial clinical experience. Radiol Med 2015;120:338-44. [Crossref] [PubMed]
- Wang H, Ke M, Li W, et al. Chinese expert consensus on diagnosis and management of acquired respiratory-digestive tract fistulas. Thorac Cancer 2018;9:1544-55. [Crossref] [PubMed]
- Jones C, Crerar-Gilbert AJ, Madden BP. Anaesthesia for endobronchial intervention and tracheobronchial stents. Curr Anaesth Crit Care 2009;20:160-3. [Crossref]
- Herth F, Becker HD, LoCicero J 3rd, et al. Successful bronchoscopic placement of tracheobronchial stents without fluoroscopy. Chest 2001;119:1910-2. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68 Suppl 1:i1-44. [Crossref] [PubMed]


