Left heart decompression at venoarterial extracorporeal membrane oxygenation initiation in cardiogenic shock: prophylactic versus therapeutic strategy
Introduction
Venoarterial extracorporeal membrane oxygenation (VA ECMO), which employs temporary mechanical circulatory support devices, is used to achieve hemodynamic stability and maintain adequate end-organ perfusion in patients with refractory cardiogenic shock (CS). Despite its major advantages in the clinical setting and its association with significantly decreased mortality in patients with refractory CS (1-3), ECMO has some important issues that must be addressed for its effective use. ECMO has a limited effect on reducing left ventricle (LV) volume and pressure, known as “LV unloading” (4). Impaired LV unloading can cause ventricular distension and pulmonary edema and may also lead to increased LV end-diastolic pressure, myocardial ischemia, and increased mortality in CS patients who need ECMO support (5-7). In some instances, left heart decompression (LHD) to treat LV distension after its complications may be futile in this setting. Therefore, in theory, prophylactic approaches to LV unloading before LV distension in patients on VA ECMO can improve clinical outcomes. However, data on the effects of prophylactic LV decompression are limited, and most previous studies have been conducted in pediatric populations (8-10). Therefore, this study investigated the effects of concomitant percutaneous transseptal left atrial (LA) drainage for LHD at ECMO initiation on the clinical outcomes of adult patients with CS.
Methods
Study design
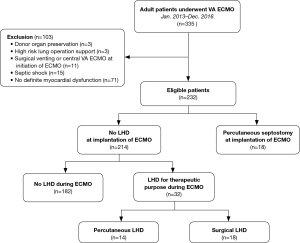
We conducted a retrospective cohort study at a single tertiary care center in Korea from January 2013 to December 2016. A total of 335 consecutive adult patients (aged ≥18 years) underwent VA ECMO during the study period. The patients who required ECMO support to improve shock conditions caused by myocardial pump failure were enrolled in the study. Exclusion criteria included ECMO for hemodynamic support for donor organ preservation, septic shock, cardiac arrest due to non-cardiovascular or unknown causes, cardiac tamponade, pulmonary thromboembolism, acute aortic syndrome, or idiopathic ventricular arrhythmia; ECMO support only during an operation; and central ECMO with a vent used from the beginning of ECMO support. Among the 232 eligible patients, 18 underwent percutaneous LA decompression at ECMO initiation, and 32 patients underwent LHD to treat complications of impaired LV unloading (Figure 1).
Implementation and management of ECMO
Patients with profound CS were considered candidates for ECMO, and ECMO initiation was determined by a cardiologist or a cardiovascular surgeon. Our multidisciplinary ECMO team did daily rounds and assessed circuit state, development of ECMO-associated complications, and possibility of weaning. Arterial, central venous, and/or pulmonary artery catheters were used for continuous hemodynamic monitoring. Pump blood flow rate was adjusted to maintain mean arterial pressure at 60–90 mmHg and adequate tissue perfusion. Intravenous fluid, blood product, inotropes, vasopressors, or vasodilators were infused as needed. Echocardiography was performed to monitor cardiac function. An ECMO weaning trial was considered when patients were hemodynamically stable with or without a low level of pharmacologic support and with adequate natural lung oxygenation capacity. Successful weaning was defined as weaning from ECMO followed by survival without reinsertion beyond 24 h. For the patients in whom ECMO weaning was impossible, bridging to cardiac replacement therapy, such as a left ventricular assist device (LVAD) or heart transplantation, was considered.
Indication and technique of percutaneous transseptal LA drainage
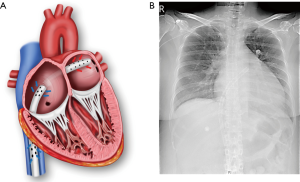
There was no predefined indication for concomitant percutaneous transseptal LA drainage for LHD at ECMO initiation. Prophylactic LHD was considered when the patient was deemed at high risk of problems related to impaired LV unloading based on clinical factors such as the severity of ventricular dysfunction and fluid balance and absence of reversible causes of myocardial dysfunction. LHD was generally not performed when the cause of cardiac failure was corrected or would be corrected soon. Typically, CS related to acute coronary syndrome was not indicated for prophylactic LHD. The procedure was performed in the catheterization laboratory. In relatively stable patients, we attempted to perform transseptal LA cannulation first. Via the femoral vein, an 8-Fr Mullins sheath (Medtronic, Inc., Minneapolis, MN, USA) was advanced over a guide wire to the superior vena cava. A Brockenbrough transseptal needle (St. Jude Medical, Inc., St Paul, MN, USA) was introduced into the sheath, and the whole unit was withdrawn. The optimal puncture site was determined with fluoroscopic and transesophageal echocardiographic guidance, and transseptal puncture was performed. After puncture site dilation with a percutaneous mitral valvuloplasty dilator, a 21–25-Fr cannula with multiple side holes was positioned in the LA. The LA drain was subsequently incorporated into the venous limb of the circuit. If the patient became very unstable, we performed standard VA ECMO and proceeded with the transseptal approach. Seventeen (53.1%) of 32 patients who underwent percutaneous transseptal LA drainage did not require a separate right atrial drainage cannula. In general, a transseptal 24-Fr multiple-side-hole cannula with a length of >55 cm could effectively decompress both the right and left heart (Figure 2). Transaortic pig tail drainage was not favored due to limited unloading capacity. Surgical decompressive procedure was performed when catheter based LHD failed or was contraindicated.
Data collection and clinical outcomes
The clinical and laboratory data collected on the day of ECMO implantation were retrospectively obtained from a thorough review of the electronic medical records. The primary outcome was 30-day mortality after ECMO initiation. Secondary outcomes included the myocardial recovery rate, cardiac replacement therapy rate, ECMO support duration, and ECMO-associated complications including limb ischemia, cannula insertion site bleeding, cannula insertion site infection, ischemic or hemorrhagic stroke, gastrointestinal bleeding, or other technical problems. Clinical outcomes were identified by the hospital medical chart including laboratory, endoscopic, and radiologic data. To determine whether patients died, we referred to the Korean national database using a citizen registration number unique to each individual. The median follow-up, as of July 17, 2017, was 202 [33–737] days after ECMO initiation. The Institutional Review Board at Samsung Medical Center approved the study protocol and waived the requirement for informed consent (No. 2018-07-001).
Statistical analysis
Categorical variables are presented as numbers and percentages; continuous variables are presented as median with interquartile ranges. To compare the characteristics and clinical outcomes between the therapeutic and prophylactic LHD groups, the χ2 or Fisher’s exact test and the Mann-Whitney test were used for categorical variables and continuous variables, respectively, when applicable. P<0.05 in the two-tailed test was considered statistically significant. We used SPSS version 23.0 (SPSS Inc., Chicago, IL, USA) for all statistical analyses.
Results
Baseline clinical characteristics
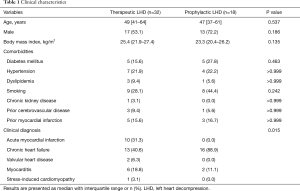
The baseline clinical characteristics of the 50 CS patients who received ECMO support with an LHD procedure are shown in Table 1 (therapeutic LHD group, n=32; prophylactic LHD group, n=18). Age, sex, and comorbidities were not significantly different between the groups. In terms of the clinical diagnosis that led to CS, therapeutic LHD was performed in patients with chronic heart failure (40.6%), acute myocardial infarction (31.3%), myocarditis (18.8%), valvular heart disease (6.3%), and stress-induced cardiomyopathy (3.1%), while prophylactic LHD was performed in patients with chronic heart failure (88.9%) and myocarditis (11.1%) (P=0.015).
Full table
Laboratory and echocardiographic characteristics prior to ECMO
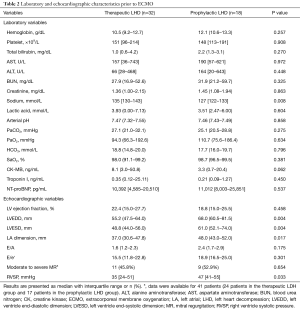
Complete blood count, liver and kidney function test, and arterial blood gas analysis results prior to ECMO implementation were similar between groups (Table 2). Median sodium level was lower in the prophylactic LHD group (135 mmol/L) than in the therapeutic LHD group (127 mmol/L) (P=0.008). The median LV ejection fraction prior to ECMO initiation was 22.4% and 18.8% in the therapeutic and prophylactic LHD groups, respectively (P=0.458). LV and LA chamber sizes were significantly larger in the prophylactic LHD group: LV end-diastolic dimension, 55.2 vs. 68.0 mm, P=0.004; LV end-systolic dimension, 48.8 vs. 61.0 mm, P=0.004; and LA dimension, 37.0 vs. 48.0 mm, P=0.017. About half of the patients showed moderate to severe mitral regurgitation on transthoracic echocardiogram. Median right ventricular systolic pressure, calculated using tricuspid regurgitation peak velocity, was 35 and 47 mmHg in the therapeutic and prophylactic LHD groups, respectively (P=0.033).
Full table
Treatment characteristics in the intensive care unit
In the therapeutic LHD group, median time interval from ECMO initiation to LHD procedure was 38.8 hours, and LHD was performed using the percutaneous and surgical approaches in 43.8% and 56.2% of this group, respectively (Table 3). All patients used inotropes or vasopressors for hemodynamic support. The proportions of patients requiring mechanical ventilation (87.5% vs. 77.8%, P=0.436) and continuous renal replacement therapy (62.5% vs. 44.4%, P=0.217) were numerically higher in the therapeutic LHD group, although the difference was not statistically significant.

Full table
Clinical outcomes
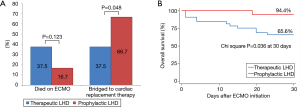
Overall, 15 (30.0%) patients died while on ECMO, and 3 (6.0%) were weaned from ECMO but died while hospitalized (Table 4). None of the patients required additional procedures or surgery for inadequate LA decompression. Among the 20 (62.5%) patients in the therapeutic LHD group and 15 (83.3%) in the prophylactic LHD group who were successfully weaned from ECMO, one in each group needed ECMO again during the same hospitalization period (Figure 3). The recovery rates were 25.0% and 16.7% in the therapeutic and prophylactic LHD groups, respectively (P=0.724). The prophylactic LHD group (3 patients received LVAD and 9 patients received heart transplant) had significantly higher rate of bridging to cardiac replacement therapy than the therapeutic LHD group (1 patient received LVAD and 11 patients received heart transplant) (66.7% vs. 37.5%, P=0.048). Of the 20 patients who received heart transplant, 6 patients (2 in therapeutic LHD and 4 in prophylactic LHD) were listed for cardiac transplantation prior to ECMO insertion, and 7 patients (3 in therapeutic LHD and 4 in prophylactic LHD) decided to do transplantation when the ECMO is implemented. In the remaining seven patients (6 in therapeutic LHD and 1 in prophylactic LHD), ECMO weaning was not possible due to insufficient improvement of myocardial dysfunction and heart transplantation was determined within one month after ECMO insertion. The 30-day mortality rate was also lower in prophylactic LHD group than the therapeutic LHD group (5.6% vs. 34.4%, P=0.036). The 90-day mortality was 43.8% (n=14) and 22.2% (n=4) in therapeutic LHD and prophylactic LHD, respectively (P = 0.128): 12 patients died of progression of cardiac insufficiency (11 in therapeutic LHD and 1 in prophylactic LHD), 4 patients died of ECMO-related complications (2 in therapeutic LHD and 2 in prophylactic LHD), and one patient in therapeutic LHD died of sepsis. The cause of death was not determined in one patient. The incidence of ECMO-related complications was similar between the groups. Three patients who attempted percutaneous LHD suffered an aortic injury during the septal puncture and required repair operation.

Full table
Discussion
In the present study, we reviewed the experience of prophylactic percutaneous transseptal LA drainage at ECMO initiation in adult patients with CS. Among the 335 patients treated with VA ECMO, 58 (17.3%) underwent LV decompression, including 32 patients who required LHD for therapeutic purposes. Prophylactic LHD was primarily performed in patients with chronic heart failure who were diagnosed with dilated cardiomyopathy. We also performed prophylactic LHD in a patient with very severe LV dysfunction who may have been unable to overcome the increased afterload caused by VA ECMO. Overall, the prophylactic LHD group had higher rate of bridging to cardiac replacement therapy and significantly lower 30-day mortality rate than the therapeutic LHD group. In this study, we showed the feasibility of using a single transseptal drainage cannula with VA-ECMO (Figure 2). Although the TandemHeart device has been used as a peripheral temporary left ventricular assist device, it cannot decompress the right heart and requires a specialized cannula for LA drainage. In our institution, various sizes of femoral venous cannulas (21–25-Fr) with sufficiently long side holes, generally 6 cm, are used for biatrial decompression. Because the membrane oxygenator is always applied, the blood returning to the patient is well oxygenated.
Complications caused by impaired LV unloading in patients on ECMO are not only inevitable, but can also be fatal (5-7). Therefore, various methods including venting cannula insertion (11-13), balloon atrial septostomy (14-16), stent implantation (9), transaortic pigtail catheter insertion (17), placement of a cannula in the pulmonary artery trunk (18) and concomitant use of other mechanical circulatory support devices such as the intra-aortic balloon pump (IABP) (19) or Impella (20) have been used for LV decompression. In our study, the percentage of therapeutic LHD did not differ according to the use of the IABP (15.4% in patients who concurrently used IABP and ECMO and 14.9% in patients who used ECMO only). The incidence of LHD in patients on VA ECMO is reported to be 10.5–20.0% (8-10,21). Percutaneous methods can effectively decompress the LV while avoiding surgical complications and are advantageous in that they can be performed at bedside under transesophageal echocardiographic guidance (15,16,22). However, the percutaneous method via trans-septal placement of a LA cannula is not direct LV decompression and may be accompanied by a decrease in aortic valve opening and blood flow out of the ventricle due to the reduced LV preload. Therefore, appropriate monitoring for aortic valve opening and stasis within the LV is needed (23). In our study, percutaneous and surgical techniques were used in 32 and 29 of the 335 patients on ECMO, respectively. All cases of surgical decompression before LV distension were performed in patients with central ECMO.
No studies have investigated the prophylactic LHD strategy and its outcomes. Kotani et al. demonstrated that early decompression was not associated with improved survival, ECMO weaning, or myocardial recovery in their study involving 23 pediatric patients including 19 postcardiotomy patients and 4 patients with cardiomyopathy who underwent VA ECMO (8). Eastaugh et al. studied 44 pediatric patients (22 with myocarditis and 22 with non-myocarditis cardiac disease) who underwent percutaneous LA decompression on top of ECMO and showed that the timing of LA decompression after ECMO deployment did not influence survival to hospital discharge (9). In a recent study of 51 pediatric patients with various diagnoses including sepsis and cardiac disease, there was no association between elective LHD at ECMO initiation and improved survival compared to emergency LHD after LV distension complications. However, elective LHD did result in a shorter ECMO support duration, especially in non-cardiac patients (10).
Although our study did not demonstrate any benefit of prophylactic LHD in terms of myocardial recovery, it identified an association between prophylactic LHD and a higher rate of successful bridging to heart transplantation or a LVAD and improved survival rate. This might be partially related to the characteristics of the enrolled patients. Previous studies were conducted in pediatric populations, and a large number of the patients had diagnosed or suspected myocarditis or congenital heart disease following cardiac surgery. In the present study, only adult patients with cardiac disease were included, and chronic heart failure, which may necessitate prolonged mechanical circulatory support or heart transplantation due to severe myocardial dysfunction and a decreased likelihood of myocardial recovery, was considered the main indication for prophylactic LHD. Regarding bridge to cardiac replacement therapy, adequate ECMO support may have been achieved with greater stability in the prophylactic LHD group because there was no concern regarding the inherent risk of LV loading when high ECMO flow is required for hemodynamic support. This may have influenced organ perfusion and the occurrence of organ dysfunction, two critical factors in determining heart transplantation and prognosis after transplantation (24,25). Median time interval from ECMO initiation to LHD was 38.8 hours in the therapeutic LHD group, and follow-up lactic acid levels were significantly lower in the prophylactic LHD group than in the therapeutic LHD group during this time period.
The present study has several limitations. First, we cannot rule out the possibility of selection bias because we included only those patients with appropriate indications for prophylactic LHD. As we described our general indication of prophylactic LHD, we did not routinely perform it. We believe primary pathophysiology of LV distension was severity of LV dysfunction which cannot overcome afterload i.e., aortic blood pressure generated by ECMO flow and vascular resistance. Because cause-corrected myocardial dysfunction generally recovers quickly at least as much as it decompresses itself, our strategy was made by cardiovascular physiology and our experiences. Second, our study was a retrospective cohort study in a single center with a small proportion of patients undergoing ECMO; therefore, the findings were likely underpowered. Furthermore, the timing of implementation and management of ECMO, indications for prophylactic LHD, and concomitant medical therapy, mechanical ventilation, and renal replacement therapy during ECMO were left to the individual physician’s discretion without an established protocol. Further prospective randomized controlled studies with well-established protocols are needed to list the recommendations for candidate of prophylactic LHD and to elucidate the proper timing of LHD in patients on ECMO. Third, because percutaneous transseptal LA drainage was performed by skilled interventional cardiologists and an ECMO team including an experienced perfusionist routinely participated in the management of ECMO, the generalizability of our findings may be limited. Fourth, the effects of prophylactic LHD on hemodynamic parameters such as cardiac index, pulmonary capillary wedge pressure, or LV end-diastolic pressure were not evaluated. Last, we were unable to demonstrate significant improvements in longer-term (90-day) survival despite the positive clinical impact on 30-day mortality. Further investigation in a larger study population is warranted.
Conclusions
Concomitant percutaneous transseptal LA drainage at ECMO initiation was associated with decreased early mortality and a higher likelihood of successful bridging to cardiac replacement therapy. Preventive or early LHD may improve clinical outcomes in patients at high risk of LV distension. However, our study has several biases inherent in retrospective observational study design. Prospective randomized controlled studies are needed to make reasonable recommendations for prophylactic LHD.
Acknowledgments
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2019R1F1A1061711).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Review Board at Samsung Medical Center approved the study protocol and waived the requirement for informed consent (No. 2018-07-001).
References
- Werdan K, Gielen S, Ebelt H, et al. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014;35:156-67. [Crossref] [PubMed]
- Takayama H, Truby L, Koekort M, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant 2013;32:106-11. [Crossref] [PubMed]
- Hryniewicz K, Sandoval Y, Samara M, et al. Percutaneous Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock Is Associated with Improved Short- and Long-Term Survival. ASAIO J 2016;62:397-402. [Crossref] [PubMed]
- Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol 2015;65:e7-e26. [Crossref] [PubMed]
- Kawashima D, Gojo S, Nishimura T, et al. Left ventricular mechanical support with Impella provides more ventricular unloading in heart failure than extracorporeal membrane oxygenation. ASAIO J 2011;57:169-76. [Crossref] [PubMed]
- Lucas SK, Schaff HV, Flaherty JT, et al. The harmful effects of ventricular distention during postischemic reperfusion. Ann Thorac Surg 1981;32:486-94. [Crossref] [PubMed]
- Bavaria JE, Furukawa S, Kreiner G, et al. Effect of circulatory assist devices on stunned myocardium. Ann Thorac Surg 1990;49:123-8. [Crossref] [PubMed]
- Kotani Y, Chetan D, Rodrigues W, et al. Left atrial decompression during venoarterial extracorporeal membrane oxygenation for left ventricular failure in children: current strategy and clinical outcomes. Artif Organs 2013;37:29-36. [Crossref] [PubMed]
- Eastaugh LJ, Thiagarajan RR, Darst JR, et al. Percutaneous left atrial decompression in patients supported with extracorporeal membrane oxygenation for cardiac disease. Pediatr Crit Care Med 2015;16:59-65. [Crossref] [PubMed]
- Hacking DF, Best D, d'Udekem Y, et al. Elective decompression of the left ventricle in pediatric patients may reduce the duration of venoarterial extracorporeal membrane oxygenation. Artif Organs 2015;39:319-26. [Crossref] [PubMed]
- Cheung MM, Goldman AP, Shekerdemian LS, et al. Percutaneous left ventricular "vent" insertion for left heart decompression during extracorporeal membrane oxygenation. Pediatr Crit Care Med 2003;4:447-9. [Crossref] [PubMed]
- Aiyagari RM, Rocchini AP, Remenapp RT, et al. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med 2006;34:2603-6. [Crossref] [PubMed]
- Hlavacek AM, Atz AM, Bradley SM, et al. Left atrial decompression by percutaneous cannula placement while on extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2005;130:595-6. [Crossref] [PubMed]
- Seib PM, Faulkner SC, Erickson CC, et al. Blade and balloon atrial septostomy for left heart decompression in patients with severe ventricular dysfunction on extracorporeal membrane oxygenation. Catheter Cardiovasc Interv 1999;46:179-86. [Crossref] [PubMed]
- Johnston TA, Jaggers J, McGovern JJ, et al. Bedside transseptal balloon dilation atrial septostomy for decompression of the left heart during extracorporeal membrane oxygenation. Catheter Cardiovasc Interv 1999;46:197-9. [Crossref] [PubMed]
- Koenig PR, Ralston MA, Kimball TR, et al. Balloon atrial septostomy for left ventricular decompression in patients receiving extracorporeal membrane oxygenation for myocardial failure. J Pediatr 1993;122:S95-9. [Crossref] [PubMed]
- Barbone A, Malvindi PG, Ferrara P, et al. Left ventricle unloading by percutaneous pigtail during extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg 2011;13:293-5. [Crossref] [PubMed]
- Fouilloux V, Lebrun L, Mace L, et al. Extracorporeal membranous oxygenation and left atrial decompression: a fast and minimally invasive approach. Ann Thorac Surg 2011;91:1996-7. [Crossref] [PubMed]
- Yang F, Jia ZS, Xing JL, et al. Effects of intra-aortic balloon pump on cerebral blood flow during peripheral venoarterial extracorporeal membrane oxygenation support. J Transl Med 2014;12:106. [Crossref] [PubMed]
- Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella((R)) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404-12. [Crossref] [PubMed]
- Booth KL, Roth SJ, Perry SB, et al. Cardiac catheterization of patients supported by extracorporeal membrane oxygenation. J Am Coll Cardiol 2002;40:1681-6. [Crossref] [PubMed]
- Swartz MF, Smith F, Byrum CJ, et al. Transseptal catheter decompression of the left ventricle during extracorporeal membrane oxygenation. Pediatr Cardiol 2012;33:185-7. [Crossref] [PubMed]
- Rao P, Khalpey Z, Smith R, et al. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ Heart Fail 2018;11:e004905. [Crossref] [PubMed]
- Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant 2006;25:1024-42. [Crossref] [PubMed]
- Kilic A, Allen JG, Weiss ES. Validation of the United States-derived Index for Mortality Prediction After Cardiac Transplantation (IMPACT) using international registry data. J Heart Lung Transplant 2013;32:492-8. [Crossref] [PubMed]