
Intracavitary chemotherapy with epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) is not superior to TKI monotherapy in controlling malignant pleural effusion recurrence in EGFR-mutated lung cancer patients
Introduction
Lung cancer is a leading cause of cancer deaths worldwide (1). Malignant pleural effusions (MPEs) is a common and devastating complication in patients with non-small cell lung cancer (NSCLC). Approximately 15% of lung cancer patients present with MPEs at the time of initial diagnosis and one-half of patients subsequently develop MPEs (2,3). NSCLC patients with MPEs usually have advanced disease and a poor prognosis (4). There are currently several management options for MPEs, including chemical pleurodesis [bleomycin (BLM) or cisplatin] with chest tubes or talc pleurodesis, medical thoracoscopy, pleuroperitoneal shunts, and chronic indwelling pleural catheters (5-7). Cisplatin is the most commonly used for the treatment of NSCLC. It is thus the most frequently used drug for intrapleural therapy of pleural effusions caused by NSCLC. BLM was chosen because it is one of the most frequently used agents and is considered to have high efficacy, low toxicity and high availability. None of these management options, however, are satisfactory in controlling pleural effusions and some of these options have additional adverse reactions.
With advances in molecular detection, the epidermal growth factor receptor (EGFR) gene mutation has been shown to be a major driver gene in Asian patients with NSCLC (8). Lung adenocarcinoma patients with MPEs have a higher EGFR mutation rate (approximately 70%) (9). Sensitizing EGFR mutations are associated with a better response to first-generation EGFR-tyrosine kinase inhibitors (EGFR-TKIs) than standard chemotherapy, such as gefitinib or erlotinib (10). Icotinib is also a first-generation EGFR-TKI which has been shown to have efficacy as therapy for advanced NSCLC with EGFR-activating mutations (11).
Talc pleurodesis to control MPE is not approved in China. Thus, intrapleural chemotherapy is usually administered to patients with MPE, such as cisplatin or BLM. Although these modes of therapy can alleviate symptoms in some patients, the relapse rate is as high as 50% (12). Pleurodesis with chemotherapy has some side effects, such as nausea, vomiting, chest pain, fever, and hematologic toxicity (6). Therefore, we suggest that EGFR-TKIs without intrapleural chemotherapy have a similar curative effect in controlling MPE, while decreasing adverse events in patients with EGFR-mutated NSCLC. There are few studies which have compared EGFR-TKIs with or without intrapleural chemotherapy to control MPE in patients with NSCLC. We conducted a retrospective study and collected MPEs to analyze gene status and used part of the data to investigate if TKIs alone are effective in patients with EGFR-mutated NSCLC and MPEs and are associated with fewer adverse effects in preventing re-accumulation of MPEs.
Methods
Study populations
We retrospectively collected NSCLC patients harboring EGFR mutations with MPEs treated at the Zhejiang Cancer Hospital between January 2014 and May 2017. The study protocol was approved by our Institutional Review Board (2014-03-32).
The eligible criteria were as follows: histologically- or cytologically-confirmed NSCLC; EGFR mutations; moderate-to-massive MPEs at the time of initial diagnosis in need of clinical treatment; MPEs shown by pleural effusion cytology of pleural effusions with confirmed malignant cells; received EGFR-TKI (gefitinib, icotinib, or erlotinib) treatment; and Eastern Cooperative Oncology Group Performance Status score (ECOG PS) of 0–3. Data on patient gender, age, smoking status, baseline ECOG PS at the start of treatment with EGFR-TKIs, the type of EGFR mutations, the type of EGFR-TKIs administered (icotinib, gefitinib, or erlotinib), the time-to-effusion recurrence and tumor or other lesion recurrence, and overall survival (OS) were recorded.
EGFR mutation analyses
The specimens from each patient for genetic testing in this study were obtained from primary tumors or metastatic sites by diagnostic or surgical procedures. All samples consisted of paraffin-embedded materials. DNA was extracted from tumor tissues (FFPE DNA kit; AmoyDx, Xiamen, China) and the ARMS assay was used to detect EGFR mutation status (EGFR 18–21). Using real-time PCR (AmoyDx), analyses of genomic DNAs extracted from cell pellets were performed for detection of EGFR mutations of MPE samples in some patients.
Treatment and response assessment
EGFR-TKI with gefitinib (250 mg/day), erlotinib (150 mg/day), or icotinib (125 mg/3 times a day) was administered for NSCLC patients until progression or intolerable adverse effects. Intrapleural therapy drugs included BLM and cisplatin. After draining the pleural fluid by thoracentesis, 60 mg of cisplatin or BLM monotherapy [1 mg/kg (maximum, 60 mg/body)] was administered intrapleurally according to their attending doctor and the drain was clamped after the installation of chemotherapeutic. After intrapleural administration, the patients were asked to turn over every 15 min to facilitate full access of the delivered drugs to the chest wall.
Tumor response was evaluated by computed tomography (CT) every 4–8 weeks according to the RECIST 1.1 (13). Recent objective responses were determined according to a previous study involving the effect of MPE treatment (14). MPEs were also evaluated by CT every 4–8 weeks. Complete remission (CR) was considered when the accumulated fluid had disappeared and was stable for at least 4 weeks. Partial remission (PR) was considered when >50% of the accumulated fluid had disappeared, symptoms had improved, and the remaining fluid had failed to increase for at least 4 weeks. Remission not obvious (NC) was considered when <50% of the accumulated fluid had disappeared. Disease progression was considered when the accumulated fluid had increased. The total efficiency was calculated by taking the sum of CR + PR. Progression in non-target lesions was defined as progression of pre-existing lesions, progression due to new lesions in the thoracic cavity, new lesions beyond the thoracic cavity, or a new MPE (15). The rapid progression of MPE was defined as hospitalization or death attributable to disease progression by the research according to Chaft et al. (16). Rapid progression included preceding symptoms of disease progression and hospitalization for drainage of an MPE. Adverse reactions were evaluated by the Common Toxicity Evaluation Criteria (CTC) according to the National Cancer Institute (NCI).
Follow-up evaluation
Intrapleural progression-free survival (iPFS) was evaluated from the date of the EGFR-TKI treatment to the date of confirming re-accumulation of MPE progression, the death from any cause, or the last follow-up visit. Extrathoracic PFS (ePFS) was evaluated from the date of the EGFR-TKI treatment to the date of confirming lesion progression, the death from any cause, or the last follow-up visit. Progression-free survival (PFS) was defined from the date of EGFR-TKI treatment to the date of confirming disease progression (intrapleural progression or extrathoracic progression), death from any cause, or the last follow-up visit. OS was measured from the date of the EGFR-TKI treatment to death or the last follow-up visit. If the complete survival time of a patient was impossible to obtain or the disease did not progress, patient status was assumed as the last known survival and/or contact date. We calculated iPFS excluding intervening chemotherapy, anti-angiogenic agents, or other TKIs when MPE progressed. Patients underwent MPE drainage or continued EGFR-TKI until further disease progression and a modified therapeutic regimen.
Statistical analyses
The baseline characteristics were compared using Pearson Chi-squared or Fisher’s exact tests (when there were fewer than five expected counts in the contingency table). The Kaplan-Meier method and log-rank test were applied to evaluate the PFS and OS. Multivariate analyses were carried out by the Cox proportional-hazard model. The statistical analysis was computed using SPSS (version 19.0; SPSS, Inc., Chicago, IL, USA). Tests were two-sided and a P value <0.05 was considered statistically significant.
Results
Patient characteristics
One hundred and one patients with lung cancer presenting with MPEs at the time of diagnosis were included. All of the patients had lung adenocarcinomas and received EGFR-TKI treatment. Fifty-eight patients underwent drainage of pleural effusions and were treated with a TKI alone. Forty-three patients (42.6%) were treated with TKIs, pleural effusion drainage, and intracavitary chemotherapy. Intrapleural chemotherapy included BLM (24 patients) and cisplatin (19 patients). The median age was 57 years (range, 29–82 years). Fifty-five patients (54.5%) were female. The percentage of exon 19 deletions and exon 21 L858R mutations was 55 (54.5%) and 39 (38.6%), respectively. Eighty-one patients (80.2%) received EGFR-TKIs as first-line treatment. The patient characteristics according to TKI alone and combination therapy groups are summarized in Table 1.

Full table
Short-time effect
All 101 patients were evaluated for therapeutic efficacy. The objective response rate (ORR) was 67.2% and 53.5% in the TKI alone and combination groups (P=0.076), respectively. The disease control rate (DCR) was 86.2% and 81.4% (P=0.648), respectively.
We then evaluated MPE control in 58 patients treated with EGFR-TKIs alone; the ORR and DCR were 65.5% (38/58) and 89.7% (52/58), respectively. For the 43 patients who were administered TKIs with intrapleural therapy, the ORR was 58.1% (25/43) and the DCR was 86.0% (37/43). The ORR and DCR were not significantly different between the two groups. Table 2 shows the general response of all the patients treated with a TKI alone and intrapleural chemotherapy combined with a TKI.

Full table
In addition, there were 55 patients with EGFR 19 deletions and 39 patients with EGFR 21 L858R mutations. The ORR and DCR for exon 19 deletions and L858R mutations were 65.5% and 61.5% (P=0.697), and 82.1% and 85.5% (P=0.731), respectively.
Long-time effect
Although all of the patients underwent response evaluations after 2 months treatment, 16 patients did not receive radiographic assessments in our hospital, including 9 patients received TKI alone and 7 patients in TKI plus intrapleural drugs. Therefore, we analyzed 85 patients and the median PFS was 10.3 months The PFS in the TKI alone (49 patients) and TKI plus intrapleural drug (36 patients) groups was 10.3 and 9.9 months, respectively (P=0.746). Multivariate analyses of PFS was performed for 85 patients. The results showed that no factor, including gender (P=0.605), smoking status (P=0.108), performance status (P=0.081), TKI treatment line (P=0.570), and the therapeutic method for controlling MPEs (P=0.744), significantly influenced the PFS.
We separately assessed iPFS and ePFS in the two groups. The iPFS was 11.9 vs. 12.7 months for the TKI and combined groups (P=0.654), showing no additive effect of intrapleural treatment in preventing MPE re-accumulation in EGFR-mutated patients receiving TKI therapy. The ePFS was not significant between the 2 groups (10.6 vs. 11.3 months, P=0.785).
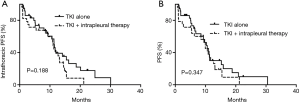
In addition, among these patients, 20 had extrathoracic tumor progression, but MPE remained well-controlled, then the drug treatment regimen was changed. The change in drugs influenced the iPFS. The remaining 65 patients had simultaneous tumor progression and MPE or stable disease. Therefore, we assessed the iPFS and PFS of the 65 patients. The iPFS was 11.4 and 11.0 months for the TKI and combination groups, respectively (P=0.188; Figure 1A). The PFS was also not significantly different between the two groups (10.3 vs. 9.9 months, P=0.347; Figure 1B).
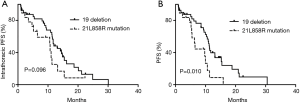
Among the 65 patients, we compared the PFS between patients with EGFR 19 deletions (40 patients) and EGFR 21 L858R mutations (19 patients). The results showed that the median iPFS was 12.0 and 10.7 months for the 2 gene types in NSCLC patients with MPEs, respectively (P=0.096; Figure 2A); however, the ePFS in the exon 19 deletion group had a longer PFS than the L858R mutation group (11.2 vs. 6.5 months, P=0.010; Figure 2B).
According to last follow-up data (30 August 2017), including outpatient visits or telephone follow-up, 5 patients were lost to follow-up. The OS was 24.9 and 22.6 months in the TKI alone and TKI plus intrapleural drugs, respectively (P=0.543).
Clinical progression of EGFR-TKI failure
We analyzed the patterns of first disease progression in 85 patients. Seventy patients had progressive disease (36 in the TKI alone group and 34 in the combination group) and 15 maintained control of disease. Among the 70 patients in the TKI alone and combination groups the rates of happening intracavitary progression were 27.8% (10/36) and 29.4% (10/34), respectively (P=0.880).
In addition, we compared the rates of rapid progression for MPE in the two groups. Rapid progression included symptomatic disease flares and progression of MPE necessitating hospitalization for drainage. In the TKI alone and combined treatment groups, the progression rate was 44.4% (16/36) and 38.2% (13/34), respectively (P=0.900).
Evaluation of adverse reactions
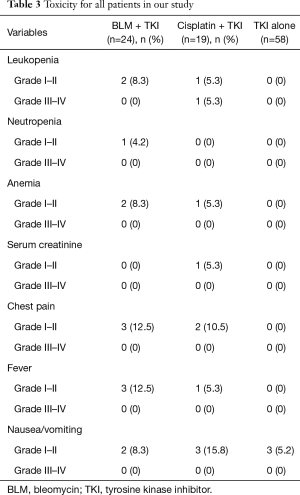
The toxicities for intrapleural therapy and TKI alone groups are listed in Table 3. Hematologic toxicity occurred in the intrapleural chemotherapy treatment. Grade 4 toxicities did not occur in all patients. Chest pain occurred mainly in the chemotherapy arm. Fever and nausea/vomiting occurred most frequently in the chemotherapy group. We analyzed statistically significant difference between TKI alone group and TKI plus intrapleural drugs group. There was a statistically significant difference in leukopenia (P=0.029), chest pain (P=0.011) and fever (P=0.029). There was no statistically significant difference in neutropenia (P=0.424), anemia (P=0.072), serum creatinine (P=0.424) or nausea/vomiting (P=0.275).
Full table
Discussion
Our findings indicate that EGFR-TKI therapy without intra-pleural chemotherapy controls MPEs and the efficacy was similar with combination treatment. Our findings are the first report to demonstrate that EGFR-TKI treatment alone may be equivalent to the addition of intrapleural chemotherapy in controlling MPE recurrences in EGFR-mutated patients.
MPEs are commonly observed in lung cancer patients, especially lung adenocarcinoma and EGFR-mutated patients (17). Administration of chemotherapy drugs or biological agents into the thoracic cavity are usually used to control MPEs. Although intra-pleural therapy prevents pleural effusions, all chemotherapeutic agents are associated with side effects, such as fever, nausea, chest pain, or hematologic toxicity. Report has demonstrated that the safety of talc pleurodesis was as thoracoscopic poudrage (18), however, talc is not available commercially in China. With the emergence of targeted drugs, modes of therapy in patients with NSCLC have change. Several prospective randomized trials have demonstrated that first-generation EGFR-TKIs as first-line treatment yield a longer PFS (9–10 months) for patients with EGFR mutations than chemotherapy (19-22).
The intrathoracic ORR (65.5% vs. 58.1%, P=0.449) and the median iPFS (11.9 vs. 12.7 months, P=0.654) did not differ between the EGFR-TKI alone treatment group compared with the combination TKI and intracavitary therapy group. Therefore, we are of the opinion that intrapleural therapy will not improve efficacy in controlling MPE. A study compared the effusion recurrence rate between gefitinib and gefitinib plus pleurodesis, including minocycline or OK432 (23). The report showed that the effusion PFS was not significantly different between the non-pleurodesis (39 patients) and pleurodesis (17 patients) groups (5.0 and 4.8 months, P=0.81). Gefitinib monotherapy provided equal efficacy in effusion control compared with gefitinib plus pleurodesis treatment. Although the results were similar to our findings, the study had only 15 EGFR mutation-positive patients. In addition, another study determined if EGFR-TKI therapy for advanced lung adenocarcinoma with MPEs was effective compared with the addition of talc (24). There were 39 patients with activating EGFR mutations and the results showed that TKIs alone compared with the addition of talc pleurodesis was adequate for preventing re-accumulation of MPEs (352 vs. 298 days, P=0.59). Therefore, these reports all demonstrated that in EGFR-mutated NSCLC patients with MPEs, intrathoracic chemotherapy may not confer additional benefit in preventing MPEs.
In addition, with respect to the patterns of MPE progression, our report indicated that 27.8% of patients in the TKI group and 29.4% of patients in the combination group exhibited intracavitary progression first. The rate of rapid progression of MPEs was 44.4% (16/36) and 38.2% (13/34), respectively (P=0.900). Our study is the first to evaluate the patterns of effusion progression between EGFR-TKI and pleurodesis to date. We also demonstrated that intrapleural chemotherapy does not play an important role in controlling re-accumulation of effusions in EGFR-mutated lung cancer.
Interestingly, some studies have shown 19 deletions and EGFR 21 L858R mutations might have different molecular subtyping and the efficacy of TKIs is also different (22,25). In our study there was no statistical difference between the 19 deletion and 21L858R-mutated patients with respect to iPFS (12.0 vs. 10.7, P=0.096); however, the exon 19 deletion group had a longer PFS than the L858R mutation group (11.2 vs. 6.5 months, P=0.010). Zheng et al. (26) compared between exons 19 and 21 EGFR mutations in NSCLC patients with MPEs after TKI therapy. The 19 deletion group had a longer PFS (9.4 vs. 7.1 months, P=0.003) and OS (16.8 vs. 13.8 months, P=0.003) compared with the L858R mutation group after second-line TKI therapy. PFS was not investigated further in the intracavitary treatment group. Although the number of patients in the 21L858 mutation group was small (19 patients) in our research, we believe this group warrants further exploration.
With respect to adverse reactions, the incidence of hematologic toxicity, fever, and chest pain in the group that received intrapleural chemotherapy was apparently higher than the TKI alone group. Therefore, we considered that TKI with intrapleural treatment had an incidence of additional adverse events similar to chemotherapy.
While our study had clinical significance, it did have limitations. The number of patients may have been insufficient. The number of enrolled EGFR L858R-mutated patients was small, thus suggesting that need for a large study. In the future, a prospective randomized design to further compare the difference between TKI alone and TKI with intrapleural treatment and different types of gene mutations is needed. Furthermore, our research mainly focused on EGFR-mutated patients with MPEs and demonstrated that the effect of EGFR-TKI monotherapy to control MPE was not difference between intrapleural chemotherapy plus TKI; however, we did not confirm this possibility in EGFR-negative patients. Thus, further research to explore the effect of intrapleural drugs should be conducted.
Conclusions
For EGFR-mutated lung cancer patients with MPEs, intrapleural chemotherapy does not increase the efficacy of controlling MPEs. In addition, the incidence of adverse events due to chemotherapy may be increased.
Acknowledgments
Funding: The study was funded by Medical Scientific Research Foundation of Zhejiang Province (No. 2016KYB046 & 2019RC027).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval was obtained by the Institutional Review Board (IRB) of each investigation site (2014-03-32). As our study was non-interventional and retrospective, formal written consent from patients was not necessary.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Memon A, Zawadzki ZA. Malignant effusions: diagnostic evaluation and therapeutic strategy. Curr Probl Cancer 1981;5:1-30. [Crossref] [PubMed]
- Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985;56:905-9. [Crossref] [PubMed]
- Wang TF, Chu SC, Lee JJ, et al. Presence of pleural effusion is associated with a poor prognosis in patients with epidermal growth factor receptor-mutated lung cancer receiving tyrosine kinase inhibitors as first-line treatment. Asia Pac J Clin Oncol 2017;13:304-13. [Crossref] [PubMed]
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013;34:459-71. [Crossref] [PubMed]
- Yoshida K, Sugiura T, Takifuji N, et al. Randomized phase II trial of three intrapleural therapy regimens for the management of malignant pleural effusion in previously untreated non-small cell lung cancer: JCOG 9515. Lung Cancer 2007;58:362-8. [Crossref] [PubMed]
- Demmy TL, Gu L, Burkhalter JE, et al. Optimal management of malignant pleural effusions (results of CALGB 30102). J Natl Compr Canc Netw 2012;10:975-82. [Crossref] [PubMed]
- Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009;10:432-3. [Crossref] [PubMed]
- Wu SG, Gow CH, Yu CJ, et al. Frequent epidermal growth factor receptor gene mutations in malignant pleural effusion of lung adenocarcinoma. Eur Respir J 2008;32:924-30. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Hu X, Han B, Gu A, et al. A single-arm, multicenter, safety-monitoring, phase IV study of icotinib in treating advanced non-small cell lung cancer (NSCLC). Lung Cancer 2014;86:207-12. [Crossref] [PubMed]
- Ishida A, Miyazawa T, Miyazu Y, et al. Intrapleural cisplatin and OK432 therapy for malignant pleural effusion caused by non-small cell lung cancer. Respirology 2006;11:90-7. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Sahn SA. Management of malignant pleural effusions. Monaldi Arch Chest Dis 2001;56:394-9. [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [Crossref] [PubMed]
- Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011;17:6298-303. [Crossref] [PubMed]
- Wu SG, Yu CJ, Tsai MF, et al. Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J 2013;41:1409-18. [Crossref] [PubMed]
- Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet 2007;369:1535-9. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, ran-domised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Shi YK, Wang L, Han B, et al. First-line icotinib versus cisplatine/pemetrexed plus pemetrexed maintenance therapy in lung adenocarcinoma patients with sensitizing EGFR mutation (CONVINCE). Ann Oncol 2016;27:1229P. [Crossref]
- Chen CH, Gow CH, Yu CJ, et al. Clinical Response of Gefitinib on Malignant Pleural Effusions in Patients with Non-Small Cell Lung Cancer. Journal of Cancer Molecules 2008;4:23-8.
- Verma A, Chopra A, Lee YW, et al. Can EGFR-Tyrosine Kinase Inhibitors (TKI) Alone Without Talc Pleurodesis Prevent Recurrence of Malignant Pleural Effusion (MPE) in Lung Adenocarcinoma. Curr Drug Discov Technol 2016;13:68-76. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Zheng Z, Xie D, Su H, et al. Treatment outcome comparisons between exons 19 and 21 EGFR mutations for non-small-cell lung cancer patients with malignant pleural effusion after first-line and second-line tyrosine kinase inhibitors. Tumour Biol 2017;39:1010428317706211. [Crossref] [PubMed]


