
Dysphagia predict the response to second cycle neoadjuvant chemotherapy in first cycle no response esophageal carcinoma
Introduction
Esophageal cancer is one of the most virulent diseases and causes approximately 400,000 deaths per year worldwide (1). In spite of recent advances in perioperative management and surgical techniques, esophageal cancer remains a highly lethal malignancy with a poor prognosis. Traditionally, carcinoma of the esophagus has been treated by surgery alone, but overall 5-year survival rates are only 5–10% (2).
It has been promising that neoadjuvant chemotherapy (NAC) has successfully improved the rates of R0 resection and short-term overall survival (OS) (3), although the survival benefit of NAC for esophageal squamous cell carcinoma (ESCC) is controversial. The standard care of locally advanced resectable ESCC in Japan is NAC (4,5) and, theoretically, NAC might eradicate systemic micrometastases and improve long-term survival (6). The cisplatin-based multi-drug chemotherapy regimen [cisplatin with 5-fluorouracil (5-Fu), taxol or paclitaxel (7)], has shown promising results. Clinical response rates (RR) of up to 70% have been reported.
Although cisplatin with paclitaxel showed promising results for ESCC, approximately 30% of patients show no clinical response. Drug resistance has been one of the most important clinical problems for ESCC (8), which cannot currently be predicted before NAC. Clinically, after the first-cycle of NAC, many patients exhibit a stable disease (SD)/progressive disease (PD) response. SD patients are recommended to receive a second cycle of NAC and PD patients are recommended to undergo surgery if possible. However, we found that some PD tumors shrink after a second cycle of NAC. Some first-cycle SD tumors may develop PD after second-cycle NAC and patients may lose the chance of surgery. Thus, how to predict the response to second-cycle NAC for first-cycle SD/PD tumors is important for clinical practice. Therefore, the effect of second-cycle of NAC was evaluated and its association with the clinicopathological features was investigated.
Methods
Patients and treatments
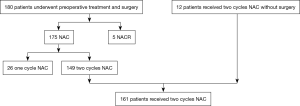
This retrospective single-center study was approved by the Institutional Review Board at the Affiliated Cancer Hospital of Zhengzhou University/Henan Cancer Hospital. The approval number is 2016ct081. From March 9, 2013 and October 24, 2016, 586 patients with ESCC underwent esophagectomy by Dr. Yin Li in our department. A total of 573 patients underwent minimally invasive esophagectomy (MIE). Among these, 180 accepted preoperative treatment, including 175 with NAC and 5 with neoadjuvant chemoradiotherapy (NACR). The patients without preoperative treatment and pN+ underwent adjuvant chemotherapy or chemoradiation postoperatively. A total of 149 of 175 patients received two cycles of NAC. During the same period, there were 12 patients who received two cycles of NAC without surgery. Finally, there were 161 patients who received two cycles of NAC delivered by the medical team of Dr. Li. The flow diagram of the patients shown in Figure 1. During this time, our principle management for local advanced ESCC was as follows: Union for International Cancer Control-tumor node metastasis (UICC-TNM) classification (9): cT4N0, cN3 or cM1lym was indicated for NACR; cN1 with any cT stage was indicated for NAC; cT2N0 was indicated for primary surgery; cT3N0 was indicated for primary surgery or NAC; pN+ was indicated for adjuvant chemotherapy; and pT4 was indicated for adjuvant chemoradiotherapy. Any cM1 except lymph node metastasis was considered a surgical contraindication.
The indicated NAC patients had to satisfy the following criteria: Eastern Cooperative Oncology Group performance status score of 0 to 2; function of the bone marrow, liver, and kidney were normal; and the patient accepted NAC. Cisplatin or nedaplatin plus paclitaxel or taxol was repeated once every 3 weeks for two cycles with a 3-week interval. Paclitaxel was administered at a dose of 175 mg/m2 by continuous infusion on days 1 and 8. Taxol was administered at a dose of 75 mg/m2 by continuous infusion on days 1 and 8. A dose of 75 mg/m2 cisplatin or nedaplatin was given by intravenous drip infusion on days 2–4. There was no death during treatment.
Three to five weeks after the second cycle of NAC the patients received surgery. All patients underwent a MIE and reconstruction by gastric conduit and at least 2-field lymph node dissections, which we reported previously (10). Persistent T4 during surgery was observed in one patient for whom R0 resection was achieved by combined resection of the adventitia of the aorta. All patients received curative resection. Disseminated metastasis before surgery was observed in one patient. Before surgery, lung metastases were also found in this patient. The total post-operation complication rate was 33%, including 15% pneumonia, 8% recurrent nerve paralysis, and no anastomotic leakage. The perioperative mortality was zero.
At the initial consultation of each hospitalization, all patients were evaluated for symptoms of dysphagia by a resident. Symptoms of dysphagia were evaluated by using a standardized dysphagia score (11). The dysphagia scores were as follows: 0= able to eat a normal diet, 1= able to swallow some solid foods, 2= able to eat semisolids only, 3= able to swallow liquids only, and 4= unable to swallow (12). If the score before any treatment minus the score 21 to 35 days after first-cycle of NAC was ≥1, it was defined as alleviated dysphagia.
Evaluation of the tumor reduction rate by Response Evaluation Criteria in Solid Tumors (RECIST) on CT scans
The enhanced 5-mm slices of the chest and upper abdominal computed tomography (CT) scans were repeated three times for NAC patients, including within 2 weeks before the first-cycle of NAC, 21 to 35 days from the first day of the first-cycle of NAC, and 21 to 35 days from the first day of the second cycle of NAC, and were evaluated according to RECIST criteria (13). For the primary tumor, we calculated only the longest diameter of the tumor and did not use the vertical section of the tumor. The first-cycle RR of the tumor was defined as: (the longest diameter of all lesions before treatment − the longest diameter of all lesions after the first-cycle of NAC)/the longest diameter of all lesions before treatment. Total RR was defined as: (the longest diameter of all lesions before treatment − the longest diameter of all lesions after the second cycle of NAC)/the longest diameter of all lesions before treatment.
Enrollment
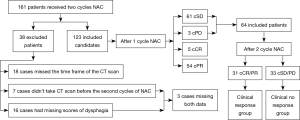
After the first-cycle of NAC, the cPR patients on CT scan would receive second cycle NAC. We were not sure whether to give the second cycle NAC for the first cycle cPD and cSD patients. Therefore we only focus on the cPD and cSD patients. There were 161 (149 with surgery, 12 without surgery) patients who received two cycles of NAC; 38 of 161 patients were excluded for various reasons. Twenty-five were excluded because the CT scan did not fulfill the criteria for this study: the timing of the CT scan was not fulfilled (18 cases) or the CT scan had not been performed before the second cycle of chemotherapy (7 cases). Sixteen patients had missing scores of dysphagia: the scores of dysphagia had not been recorded before the second cycle of NAC (15 cases) or the scores of dysphagia had not been recorded before surgery (1 case). Three patients had both missing data for the CT scan and dysphagia. Therefore, 123 patients had complete data from two cycles of NAC.
After first-cycle of NAC, 5 patients achieved a clinical CR, 54 patients achieved a clinical PR, 61 patients achieved a clinical SD, and 3 patients achieved a clinical PD. The final number of patients enrolled in this study totaled 64 first-cycle NAC-SD/PD patients.
Statistical analysis
The statistical software package SPSS 23.0 software for Windows (SPSS, Chicago, IL, USA) was used to perform statistical analyses. Chi-square tests and Fisher’s exact test were used to compare qualitative variables. The quantitative variables were compared by using Student’s t-test. The quantitative differences with multiple comparisons were analyzed by one-way analysis of variance (ANOVA). Variables in the univariate analysis with P≤0.2 were included in the multivariate analysis. For the multivariate analysis, a logistic regression with enter was performed to build the final model by all variables. A two-sided P value of <0.05 was accepted as statistically significant.
Results
Characteristics of the first-cycle SD ESCC patients are shown in Table 1. Most of the patients were male with a median age of 59.18 years (range from 42 to 76 years). The UICC-TNM classification and primary diagnosis was 10, 32 and 22 patients as cT2, cT3 and cT4, 34 and 30 patients as cN0 and cN+, and 17 and 47 patients as stages II and III, respectively. The flow diagram of the enrollment of 161 patients who received two cycles NAC was shown in Figure 2.
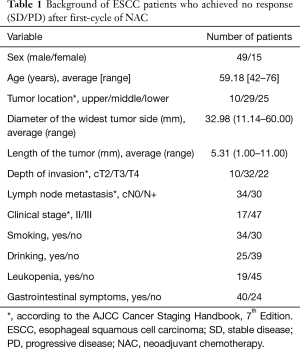
Full table
Relationship between total RR and clinicopathologic characteristics of ESCC
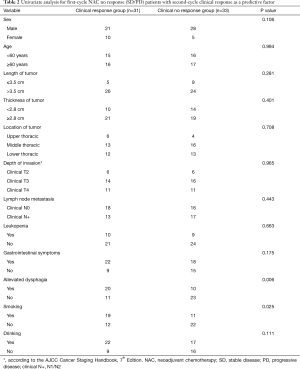
The second-cycle NAC response group showed a significant association with alleviated dysphagia after the first-cycle of NAC (P=0.006) and with a non-smoking habit (P=0.025) (Table 2). A favorable response trend showed an association with a non-drinking habit (P=0.111) and being female (P=0.106). We also examined the total clinical response after the second cycle. Thirty-one patients (48.4%) achieved a cCR/cPR 21–35 days after the second cycle of NAC and 33 patients (51.6%) achieved a cSD/cPD. The definition of clinical response/no response groups were shown in Figure 2.
Full table
Prognostic significance of total RR for first-cycle NAC-SD/PD patients
In univariate analysis, two factors were shown to be related to total RR: alleviated dysphagia (P=0.006) and smoking (P=0.025) (Table 2). Multiple logistic regression analysis suggested that alleviated only dysphagia [odds ratio (OR) 3.978; 95% confidence interval (CI), 1.335–11.856; P=0.013] was a significant independent predictor for achieving a cCR after second-cycle NAC (Table 3).
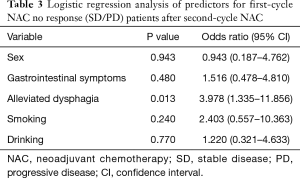
Full table
Relationship between the change of dysphagia and pathological characteristics of ESCC
There were 9 out of 64 patients without surgery. The alleviated dysphagia group showed a significant association with tumor regression grade (TRG) (P=0.019) and lymphocyte infiltration (P=0.009) (Table 4). However, there was no association with pathological T stage (P=0.498), pCR (P=0.111) or pathological N stage (P=0.341).
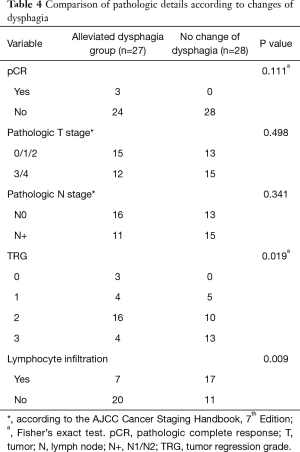
Full table
Discussion
The prediction of tumor response to chemotherapy is one of the most desirable aims in cancer treatment. Many studies have aimed to predict tumor responses to chemotherapy by using clinical and biological characteristics. At present, several investigators have suggested that molecular analysis of the biological characteristics of a tumor could be used to predict the response before treatment by biopsy samples (14); however, this approach is still far from satisfactory (15). Therefore, Weber et al. suggested that positron emission tomography (PET)/CT-evaluated changes in metabolic activity before and after treatment in early courses of chemotherapy could be used as an early predictor of response with a sensitivity and specificity of 93% and 95%, respectively (16).
However, PET/CT scans are very expensive for developing countries, where the incidence of ESCC is high. If the metabolic activity of the tumor has changed before shrinkage, it is possible that other easily available signs show up early during the course of treatment. We previously found several first-cycle no imaging response patients with alleviated dysphagia whose tumors shrank on chest CT scans after the second cycle of NAC. Based on this observation, we conducted this retrospective study with the incorporation of multiple common clinical parameters. We demonstrated the predictive value of changes of dysphagia. Tumors of the first-cycle NAC-SD/PD patients with alleviated dysphagia will probably shrink after the second-cycle of NAC. This shrinkage is easy to measure and, hence, is easy to verify in large-scale trials. It may also serve as a practical predictive marker for routine clinical use. Alleviated dysphagia before the second cycle of NAC was associated with a subsequent decrease of tumor size and pathological response of the tumor.
The most frequent and striking symptom of ESCC is dysphagia, accounting for 93.40% of patients (17). It is usually used as an early symptom for ESCC screening (18). However, we have not found any study that evaluated the predictive value of the changes in dysphagia after NAC, especially for first-cycle NAC-SD/PD patients.
Chemotherapy mainly targets rapidly dividing cells that drive growth and invasion (19). Paclitaxel targets microtubule function, which is essential for the stochastic switching between shrinkage and growth (20). Theoretically, if the chemotherapy regimen works, the tumor should shrink. A prolonged and durable SD may happen in early courses of chemotherapy and in immunotherapy (21). In early courses of chemotherapy, SD may represent primary drug resistance (20), inflammation or fibrous stroma (22).
Our data showed that alleviation of dysphagia was related to inflammatory cell infiltration (Table 4), possibly due to tumor cell degeneration and inflammatory cell infiltration, so the tumor was getting soft. Consequently, the change in dysphagia. Sometimes tumor cells may be killed but the fibrous stroma remains (22). Therefore, the loss of cellularity after NAC cannot be reflected by the same size on CT scan over a short period of time (22).
Kim et al. reported the shrinkage pattern after NAC (23). Primary breast cancers essentially melted before the tumor shrank during the early course of NAC in type III and IV patterns (23). Rajan et al. demonstrated that chemotherapy can dramatically reduce cellularity but has little effect on tumor size (24). The mechanism might be the same as in our study (i.e., an SD response on CT scan and alleviated dysphagia). Taken together, these studies could explain the imaging response to the second cycle of NAC in first-cycle SD patients with alleviated dysphagia.
Although the multi-regression analysis revealed that smoking was not a predictor of response, it was associated with an overall response in the univariate analysis and may have a significant influence on a large number of patients. Smoking is a high risk factor for ESCC (25). However, few studies have evaluated smoking as a predictive factor for NAC. In our univariate analysis, we found that smoking was a negative predictor for first-cycle NAC-SD/PD patients. The mechanism of primary drug resistance by smoking remains unknown. Smoking is associated with increased mutation burdens (25), which may be one possible reason for this observation, whereas other studies have suggested the over-expression of a DNA repair enzyme in long-term smokers (26). One or both of these mechanisms may be reasons for the failure of NAC.
Although the results of our study are encouraging, the following limitations cannot be neglected. It was a retrospective study. The change of dysphagia is not an objective indicator, although it is easy to obtain in daily practice. In future studies, we will use objective methods instead of symptoms alone. In our data, we found that alleviated dysphagia was significantly related to the TRG, the pathological response of the tumor. Heger et al. reported histopathological responders of gastric cancer after chemotherapy and showed that interim endoscopy after 6 weeks predicted response and prognosis (27). We may explore the histopathological response for clinically first-cycle CT-based SD patients to determine their individual treatment. Another potential objective measurement is esophageal manometry. There are many tools to detect the change of dysphagia objectively, such as high-resolution manometry and conventional manometry (28). For example, a patient with a history of dysphagia was found to have an abnormal high-pressure zone by high-resolution manometry of the esophagus and was diagnosed as basaloid squamous cell carcinoma (29). Thus, high-resolution manometry could show pressure plots, which may help us explore dysphagia in the future. Lastly, the CIs for the prediction of second-cycle NAC response were wide due to the small sample size.
Considerable efforts have been put into the identification of new chemotherapeutic drugs and the combination treatment of chemotherapy and radiotherapy. However, the new drugs and combinations still need to be proven in phase III clinical trials. Many patients show no response to preoperation treatment. The total clinical response rate is approximately 60% for NACR (30) and 38% for NAC (4). The prediction of an individual response is a hot topic without a clinical satisfactory rate. Therefore, it is unlikely that more effective preoperative treatments and more accurate predictions of tumor response will be acquired in the short term. In contrast, increasing numbers of studies have focused on the early identification of tumor response in early treatment courses, such as metabolic response on PET. Alleviating dysphagia may be a useful symptom to establish therapeutic regimes. First-cycle SD/PD patients who feel no change of dysphagia may undergo immediate surgery for those with potentially resectable disease or preoperation radiotherapy/definitive chemoradiation for patients with unresectable tumors. This individualized treatment may improve the prognosis of ESCC. Further objective validation of dysphagia and its prognostic value for individualized therapy should be tested in randomized trials. Alleviated dysphagia might be a useful factor to predict the response to second-cycle NAC for first-cycle SD/PD patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective single-center study was approved by the Institutional Review Board at the Affiliated Cancer Hospital of Zhengzhou University/Henan Cancer Hospital. The approval number is 2016ct081.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Talbäck M, Rosen M, Stenbeck M, et al. Cancer patient survival in Sweden at the beginning of the third millennium--predictions using period analysis. Cancer Causes Control 2004;15:967-76. [Crossref] [PubMed]
- Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2002;183:274-9. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Kidane B, Coughlin S, Vogt K, et al. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev 2015.CD001556. [PubMed]
- Ilson DH, Forastiere A, Arquette M, et al. A phase II trial of paclitaxel and cisplatin in patients with advanced carcinoma of the esophagus. Cancer J 2000;6:316-23. [PubMed]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003;22:7265-79. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Zheng Y, Li Y, Liu X, et al. A phase III, multicenter randomized controlled trial of neo-adjuvant chemotherapy paclitaxel plus cisplatin versus surgery alone for stage IIA-IIIB esophageal squamous cell carcinoma. J Thorac Dis 2017;9:200-4. [Crossref] [PubMed]
- Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med 1985;145:1443-6. [Crossref] [PubMed]
- Ripley RT, Sarkaria IS, Grosser R, et al. Pretreatment Dysphagia in Esophageal Cancer Patients May Eliminate the Need for Staging by Endoscopic Ultrasonography. Ann Thorac Surg 2016;101:226-30. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Motoori M, Takemasa I, Yamasaki M, et al. Prediction of the response to chemotherapy in advanced esophageal cancer by gene expression profiling of biopsy samples. Int J Oncol 2010;37:1113-20. [PubMed]
- Prasad V. Perspective: The precision-oncology illusion. Nature 2016;537:S63. [Crossref] [PubMed]
- Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 2001;19:3058-65. [Crossref] [PubMed]
- Jiang YX, Zhang DW, Chen Y, et al. The characteristics of oesophageal squamous cell carcinoma: an analysis of 1317 cases in southeastern China. Contemp Oncol (Pozn) 2015;19:137-41. [Crossref] [PubMed]
- Shao Y, Yu ZL, Ji M, et al. Lugol chromoendoscopic screening for esophageal dysplasia/early squamous cell carcinoma in patients with esophageal symptoms in low-risk region in China. Oncol Lett 2015;10:45-50. [Crossref] [PubMed]
- Davis W, Larionov LF. Progress in Chemotherapy of Cancer. Bull World Health Organ 1964;30:327-41. [PubMed]
- Alushin GM, Lander GC, Kellogg EH, et al. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell 2014;157:1117-29. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Zhou J, Qiao PG, Zhang HT, et al. Predicting Neoadjuvant Chemotherapy in Nonconcentric Shrinkage Pattern of Breast Cancer Using 1H-Magnetic Resonance Spectroscopic Imaging. J Comput Assist Tomogr 2018;42:12-8. [Crossref] [PubMed]
- Kim TH, Kang DK, Yim H, et al. Magnetic resonance imaging patterns of tumor regression after neoadjuvant chemotherapy in breast cancer patients: correlation with pathological response grading system based on tumor cellularity. J Comput Assist Tomogr 2012;36:200-6. [Crossref] [PubMed]
- Rajan R, Poniecka A, Smith TL, et al. Change in tumor cellularity of breast carcinoma after neoadjuvant chemotherapy as a variable in the pathologic assessment of response. Cancer 2004;100:1365-73. [Crossref] [PubMed]
- Alexandrov LB, Ju YS, Haase K, et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016;354:618-22. [Crossref] [PubMed]
- Guo M, Akiyama Y, House MG, et al. Hypermethylation of the GATA genes in lung cancer. Clin Cancer Res 2004;10:7917-24. [Crossref] [PubMed]
- Heger U, Bader F, Lordick F, et al. Interim endoscopy results during neoadjuvant therapy for gastric cancer correlate with histopathological response and prognosis. Gastric Cancer 2014;17:478-88. [Crossref] [PubMed]
- Roman S, Huot L, Zerbib F, et al. High-Resolution Manometry Improves the Diagnosis of Esophageal Motility Disorders in Patients With Dysphagia: A Randomized Multicenter Study. Am J Gastroenterol 2016;111:372-80. [Crossref] [PubMed]
- Liu R, Chu H, Xu F, et al. Esophageal cancer diagnosed by high-resolution manometry of the esophagus: A case report. Oncol Lett 2016;11:3131-4. [Crossref] [PubMed]
- Saeki H, Nakashima Y, Zaitsu Y, et al. Current status of and perspectives regarding neoadjuvant chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Surg Today 2016;46:261-7. [Crossref] [PubMed]


