|
Brief Report
Contralateral breast metastasis from pulmonary adenocarcinoma:
Two cases report and literature review
Fang-Fang Ji1, Peng Gao2, Ji-Gang Wang3, Jie Zhao3, Peng Zhao3
1Department of Ultrasound, the Qingdao Sanatorium, Qingdao 266071, China; 2Department of General Surgery, the Affiliated Hospital
of Medical College, Qingdao University, Qingdao 266003,China; 3Department of Pathology, the Affiliated Hospital of Medical College,
Qingdao University, Qingdao 266003, China
Corresponding to: Peng Zhao. Department of Pathology, the Affiliated Hospital of
Medical College, Qingdao University No. 16 Jiangsu Road, Qingdao, 266003, China.
Tel: +86-532-82911532; Fax: +86-532-82911533. Email: zhpeng17@hotmail.com.
|
|
Abstract
Carcinoma metastatic to breast from extra-mammary malignancy is rare and only accounts for 0.4-1.3% of all breast
cancer. Two rare cases of single breast metastasis from pulmonary adenocarcinoma were reported here with a brief
review of the pertinent literature. The only complaint of the these two female patients was painless breast mass found
recently. Most breast metastasis previously reported are present in the upper outer quadrant, however, in our study,
one case was found to be located in the lower inner quadrant and the other in the upper inner quadrant. Tumor cells
from breast biopsy were immune-positive for thyroid transcription factor-1. The two patients survived 5 and 8 months,
respectively, following the diagnosis of both the primary lung tumor and the breast metastasis. Breast metastasis from
lung adenocarcinoma is rare but does exist. The awareness of this possibility may help to differentiate the tumor from
primary breast carcinoma. Clinical history and immunohistochemical studies are essential to reach the final diagnosis.
Key words
Lung neoplasms; neoplasm metastasis; breast neoplasm
J Thorac Dis 2012;4(4):384-389. DOI: 10.3978/j.issn.2072-1439.2012.02.03
|
|
Introduction
Breast carcinoma is the most prevalent malignant tumor among women in the world. Carcinoma metastatic to breast, which is secondary to leukemia/lymphoma, melanoma, can originate from any type of malignancy ( 1- 5). However, carcinoma metastatic to breast from extra-mammary malignancy is rare and only accounts for 0.4-1.3% of all breast cancer. To the best of our knowledge, from 1991 to 2011 only 11 metastasis were classified as adenocarcinomas in the PubMed database ( Table 1). In the present report, two cases of pulmonary adenocarcinoma metastatic to the contralateral breast were described. Interestingly, the only present symptom was a painless breast mass.
| |
|
|
| Table 1. 11 cases of breast metastasis from pulmonary adenocarcinoma. |
| Author, year |
Age/sex |
Chief complaint |
Breast tumor size |
Primary tumor
location |
FolloNot availablew-up |
| Verger E et al.,
1992 (6) |
63/male |
A painless, hard mass
in
the left breast |
4 cm ×3.5 cm |
Right lung |
Not available |
| Lee SH et al.,
2000 (2 cases) (1) |
Not available |
Not available |
Not available |
Not available |
Not available |
| Masmoudi A et al.,
2003 (7) |
54/female |
Increasing breathlessness
and right breast swelling;
a lump in the left breast
upper quadrant without
evidence of skin or chest
wall involvement |
8 cm in diameter |
Right lower
pulmonary lobe |
Not available |
| Yeh CN, et al.,
2004 (2) |
44/female |
A tumor mass in the
medial lower quadrant
of the right breast,
superficially located at
the subcutaneous layer
without skin changes |
4 cm × 3 cm |
Not available |
Not available |
| Komorowski AL et al.,
2005 (8) |
48/not available |
Not available |
Not available |
Not available |
Not available |
| Lee AH et al., 2007 (3) |
64/female |
Not available |
Not available |
Not available |
Not available |
| Fulciniti F et al.,
2008 (4) |
59/female |
A poorly delimited
mass in the upper inner
quadrant of right breast,
with skin dimpling and
reddening |
Not available |
Right lung |
Still alive 14 months
after diagnosis |
| Klingen TA et al.,
2009 (5) |
79/female |
A left, subareolar tumor
mass |
8 cm in diameter |
Not available |
Died 1 month after
diagnosis of the
metastasis |
| Klingen TA et al.,
2009 (5) |
70/female |
A right, subareolar tumor
mass |
0.9 cm in diameter |
Not available |
Died 4 months after
diagnosis of the
metastasis |
| Maounis N et al.,
2010 (9) |
73/female |
A painless, poorly defined
mass, associated with
skin redness, in the upper
outer quadrant of the left
breast |
4.5 cm × 3.5 cm |
Left lung |
Died 6 months after
diagnosis of the
metastasis |
|
|
|
Case reports
Case 1
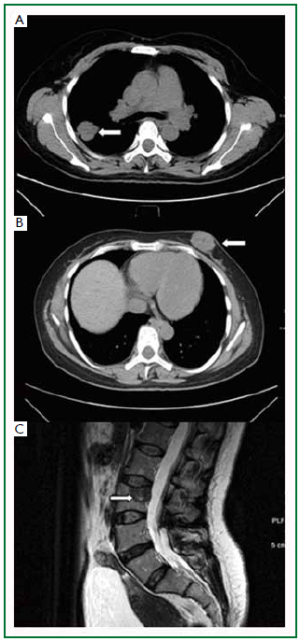
A 49-year-old, non-smoking, peasant woman presented to the general surgery clinic complaining a painless mass in her left breast for 3 months. Physical examination confirmed a round, firm, and non-tender mass located in the lower inner quadrant of her left breast. It was measured 3cm in diameter, mobile and no skin involvement. Axillary and cervical chain lymph nodes were not palpable. A chest computed tomography (CT) scan showed a round-shaped mass (3.2 cm × 3.1 cm) in the apicoposterior segment of her right lung, with a well-defined, lobulated edge and pleural indentation ( Figure 1A). No lymphadenectasis of mediastinum was observed. One subcutaneous mass was noted in the lower inner quadrant of the left breast ( Figure 1B). A review of systems did not reveal other symptoms such as dry cough, dyspnea or tachypnea. A left breast lumpectomy was performed and biopsy confirmed a poorly differentiated adenocarcinoma. A lumbar spine magnetic resonance imaging (MRI) showed another metastasis to the 3rd lumbar vertebrae ( Figure 1C). The patient refused
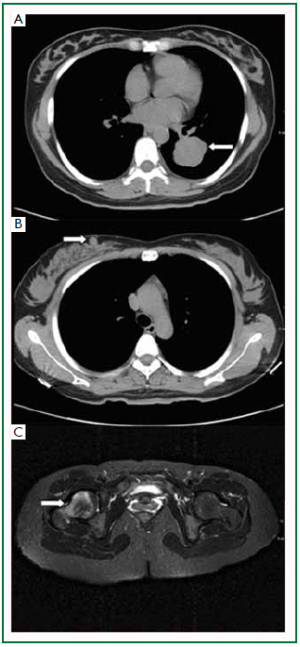
any further treatment and died 5 months after discharge. Case 2
A 40-year-old, non-smoking, business woman presented to
the general surgery clinic with a painless, palpable mass in the
right breast for 6 days. Physical examination revealed a solid,
mobile, and non-tender mass (1 cm × 1 cm) in the upper inner
quadrant of the right breast, with irregular border and without
skin involvement. Axillary and cervical chain lymph nodes
were also not palpable. The CT scan showed a soft tissue mass
measured 3.7 cm × 4.2 cm × 4.4 cm located in the inferior
lobe of her left lung, with a lobular border ( Figure 2A). Patchy
shadow, signs of bronchi blockage, and pleural displacement
were also noted in the CT images. In addition, a round soft
tissue mass with the size of 1cm in diameter was present in the
right breast ( Figure 2B), and the MRI examination of lower
limbs showed a tumor mass in the right femur ( Figure 2C). The right breast lumpectomy and bone biopsy were
performed, and the following pathology analysis revealed
poorly differentiated adenocarcinoma of the both sites.
In addition, epidermal growth factor receptor (EGFR)
was detected by immunohistochemistr y staining. The patient underwent two c ycles of Gefitinib treatment
but did not demonst rate any s ign of improvement.
The patient passed away 8 months after the diagnosis.
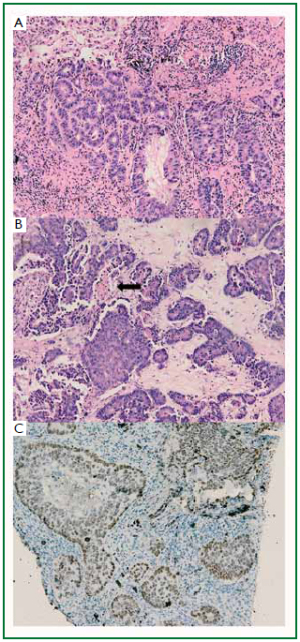
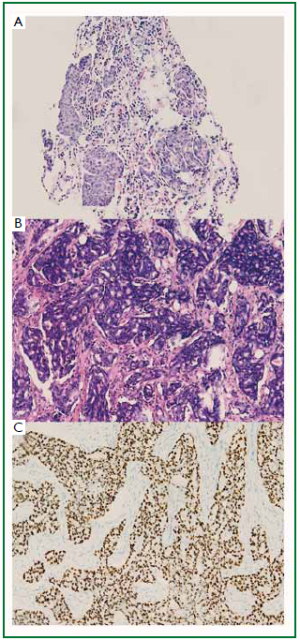
The two patients received lung mass puncture biopsy
after radiology examination. The lung mass puncture biopsy
showed poorly differentiated adenocarcinoma of the both
( Figure 3A, 4A). In case 1, the breast tumor lesion is composed
of irregular, solid malignant glands which infiltrated the dense fibrohylinized stroma. Focal necrosis was seen ( Figure 3B); however, the breast tumor of case 2 is composed of
infiltrating cribriform glands ( Figure 4B). No evidence of
in situ carcinoma or elastosis was observed in both cases.
The tumor cells demonstrated positive immunoreactivity
for thyroid transcription factor-1 (TTF-1) ( Figure 3C, 4C).
Moreover, the both tumors showed negative immunolabelling
for estrogen receptor (ER), progesterone receptor (PR),
human epidermal growth factor receptor 2 (Her-2), gross
cystic disease fluid protein 15 (GCDFP-15) and mammaglobin
(Antibodies employed in the immunohistochemistr y
were purchased f rom Santa Cruz, California , USA). |
|
Discussion
Primary breast carcinoma is the most prevalent malignant tumor
among women around the world. However, metastatic tumors in
the breast are extremely rare. Accurate differentiation of metastasis
from primary breast carcinoma is crucial in clinical practice
because the treatment and prognosis are significantly different.
The most common source of breast metastasis is the mammary
itself ( 10). Recently, breast metastasis from a wide range of extramammary
tumors has been described. It was reported that the
breast carcinoma can originate from haematological malignancies,
carcinoma of the lung, malignant melanoma, serous papillary
carcinoma of the ovary, carcinoma of the prostate, kidney and
stomach, and carcinoid tumors ( 11- 16). Georgiannos group made
a retrospectively review of more than 14,000 cases with breast
malignancies diagnosis between 1907 and 1999 (carcinoma
in situ were excluded). They found that only 60 malignancies
originated from the sites other than the breast, which made
up less than 0.5% of the total number of breast tumors. In
addition, they found that the involvement of the breast by
hematologic malignancies such as lymphoma and leukemia is
more common. When the primary sites were solid organs, small
cell carcinoma of the lung, poorly differentiated adenocarcinoma
of the stomach, renal cell carcinoma, and cutaneous malignant
melanoma were the most frequent types of source. Georgiannos
group also identified several unusual sites of origin, including the
thyroid, retina, endometrium, and pancreas ( 17). Williams et al.
investigated a series of 169 cases with metastases to the breast from
extra-mammary solid tumors ( 18). They reported that malignant
melanoma and adenocarcinoma are the two most common
histological types of breast metastases. Other tumor types include
small cell carcinoma of the lung, sarcoma, neuroendocrine tumors,
squamous cell carcinoma. Metastasis spread to unusual sites is less frequent in non-smallcell
type lung cancer (NSCLC) and the incidence of metastasis to
the breast is even lower. It was reported that the major metastasis
sites of NSCLC include liver (33-40%), adrenal glands (18-38%),
brain (15-43%), bone (19-33%), kidney (16-23%) and abdominal
lymph nodes (29%) ( 19). The unusual sites previously reported
are stomach, pancreas, small bowel, choroid plexus, muscle,
umbilicus, and the penis. The majority of breast metastasis present as palpable, rapidly
growing, well circumscribed, painless breast masses with
predilection to the upper outer quadrant ( 2, 3, 17, 18). However,
in our two cases reported here, one case was found to be located
in the lower inner quadrant and the other in the upper inner
quadrant. Unlike primary tumors, the retraction of the skin or
nipple is not demonstrated in the majority of the metastases,
despite their superficial location ( 5, 20). Breast metastasis is
associated with an extremely poor prognosis with survival period
less than one year after diagnosis ( 18). Our patients survived 5
and 8 months respectively following the diagnosis of both the
primary lung tumor and the breast metastasis. Because the imaging manifestations of the metastatic lesion
are variable, it may be extremely difficult to distinguish a breast
metastasis from a primary mammary adenocarcinoma, only
based on mammographic findings ( 17, 21). Instead, histological
indicators may help to identify the secondary tumors. Elastosis
is a consistent indicator of primary neoplasm but is rarely seen
in secondary tumors ( 22). Other clues to a metastasis rather
than primary origin include a sharp transition at the border of
the lesion and the tumor presence in the subcutaneous, rather
than parenchymal breast tissue ( 17). The absence of in situ
carcinoma strongly supports a metastatic tumor, although it
may not occur in all primary invasive carcinomas ( 9). Most
researchers agree that calcifications are extremely rare and are
seen only in the patients with metastatic papillary carcinoma
with psammoma bodies ( 9, 20, 21). Three growth patterns of
metastasis to the breast are described ( 3). The most common
one is a circumscribed nodule surrounded by normal breast
tissue. Infiltration around ducts and lobules is particularly
associated with lymphomas, leukemia and malignant melanoma.
Lymphangitis and diffuse infiltration are less common. In
our cases, breast tumor lesions are composed of irregular and
solid malignant glands infiltrating the dense, fibrohylinized
stroma. The surrounding breast parenchyma demonstrated mild
fibrocytic changes. No evidence of in situ carcinoma or elastosis
was observed. Metastasis from pulmonary adenocarcinoma might be particularly
difficult to be distinguished from primary breast carcinomas. Hence,
immunohistochemical studies may help to differentiate them. TTF-
1 has been reported positive in 93% of primary pulmonary small cell
carcinomas, and in 63% of adenocarcinomas ( 23). ER is expressed
in 80% and PR in 60% of breast carcinomas ( 24, 25). Convincing
expression of ER is largely restricted to carcinomas of the breast,
endometrium and ovary ( 26). Occasionally, tumors from other
organs also express ER, but usually it is weak and local ( 26).
GCDFP-15 is expressed in 45-53% and mammaglobin in 48-72%
of breast carcinoma ( 27- 29). In conclusion, we reported two rare cases of single breast
metastasis from a contralateral pulmonary adenocarcinoma
with only complaint of a painless mass in the breast. Unlike
most previously reported breast metastasis which are present in
the upper outer quadrant, in our study, we found one case was
located in the lower inner quadrant and the other in the upper
inner quadrant. We suppose that the primary tumor cells may
reach breast either from thoracic cavity lymphatic spread or
through thoracic duct to systemic circulation and then reached
the contralateral breast. Metastasis to the breast is rare. It can
mimic primary breast cancer in biological behavior. Sometimes
the histological characteristic is similar to a primary breast tumor
and may be difficult to be diagnosed as metastasic tumor. The
treatment and prognosis differ greatly from that of primary breast
cancer. Hence, the clinical history and immunohistochemical
studies are essential to reach the final diagnosis.
|
|
Authors' responsibility
We confirm that we have not previously published or have
not submitted the same manuscript elsewhere; we took a
significant part in the work and approved the final version of
the manuscript; we have complied with ethical standards; we
agree Pioneer Bioscience Publishing Company, to get a license
to publish the accepted article when the manuscript is accepted,
and we have obtained all necessary permissions to publish any
figures or tables in the manuscript, and assure that the authors
will pay for any necessary charges.
|
|
References
- Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the
breast: mammographic and ultrasonographic findings. J Ultrasound Med
2000;19:257-62.[LinkOut]
- Yeh CN, Lin CH, Chen MF. Clinical and ultrasonographic characteristics
of breast metastases from extramammary malignancies. Am Surg
2004;70:287-90.[LinkOut]
- Lee AH. The histological diagnosis of metastases to the breast from
extramammary malignancies. J Clin Pathol 2007;60:1333-41.[LinkOut]
- Fulciniti F, Losito S, Botti G, Di Mattia D, La Mura A, Pisano C, et al.
Metastases to the breast: role of fine needle cytology samples. Our
experience with nine cases in 2 years. Ann Oncol 2008;19:682-7.[LinkOut]
- Klingen TA, Klaasen H, Aas H, Chen Y, Akslen LA. Secondary breast
cancer: a 5-year population-based study with review of the literature.
APMIS 2009;117:762-7.[LinkOut]
- Verger E, Conill C, Velasco M, Sole M. Metastasis in the male breast from a
lung adenocarcinoma. Acta Oncol 1992;31:479.[LinkOut]
- Masmoudi A, Mathieu MC, Soria JC. Breast metastasis from lung
adenocarcinoma: a case report. Anticancer Res 2003;23:1825-6.[LinkOut]
- Komorowski AL, Wysocki WM, Mitus J. Metastasis to the breast--a clinical
challenge in outpatient. Acta Chir Belg 2005;105:59-61.[LinkOut]
- Maounis N, Chorti M, Legaki S, Ellina E, Emmanouilidou A, Demonakou
M, et al. Metastasis to the breast from an adenocarcinoma of the lung
with extensive micropapillary component: a case report and review of the
literature. Diagn Pathol 2010;5:82.[LinkOut]
- McIntosh IH, Hooper AA, Millis RR, Greening WP. Metastatic carcinoma
within the breast. Clin Oncol 1976;2:393-401.[LinkOut]
- Alva S, Shetty-Alva N. An update of tumor metastasis to the breast data.
Arch Surg 1999;134:450.[LinkOut]
- Cangiarella J, Symmans WF, Cohen JM, Goldenberg A, Shapiro RL,
Waisman J. Malignant melanoma metastatic to the breast: a report of seven
cases diagnosed by fine-needle aspiration cytology. Cancer 1998;84:160-2.[LinkOut]
- Amichetti M, Perani B, Boi S. Metastases to the Breast from Extramammary
Malignancies. Oncology 1990;47:257-60.[LinkOut]
- Topalovski M, Crisan D, Mattson JC. Lymphoma of the breast. A
clinicopathologic study of primary and secondary cases. Arch Pathol Lab
Med 1999;123:1208-18.[LinkOut]
- Majeski J. Bilateral breast masses as initial presentation of widely metastatic
melanoma. J Surg Oncol 1999;72:175-7.[LinkOut]
- Wozniak TC, Naunheim KS. Bronchial carcinoid tumor metastatic to the
breast. Ann Thorac Surg 1998;65:1148-9.[LinkOut]
- Georgiannos SN, Chin J, Goode AW, Sheaff M. Secondary neoplasms of
the breast: a survey of the 20th Century. Cancer 2001;92:2259-66.[LinkOut]
- Williams SA, Ehlers RA 2nd, Hunt KK, Yi M, Kuerer HM, Singletary SE, et
al. Metastases to the breast from nonbreast solid neoplasms: presentation
and determinants of survival. Cancer 2007;110:731-7.[LinkOut]
- Quint LE, Tummala S, Brisson LJ, Francis IR, Krupnick AS, Kazerooni EA,
et al. Distribution of distant metastases from newly diagnosed non-small
cell lung cancer. Ann Thorac Surg 1996;62:246-50.[LinkOut]
- Vizcaíno I, Torregrosa A, Higueras V, Morote V, Cremades A, Torres V, et
al. Metastasis to the breast from extramammary malignancies: a report of
four cases and a review of literature. Eur Radiol 2001;11:1659-65.[LinkOut]
- Noguera JJ, Martínez-Miravete P, Idoate F, Díaz L, Pina L, Zornoza G,
et al. Metastases to the breast: a review of 33 cases. Australas Radiol
2007;51:133-8.[LinkOut]
- Hajdu SI, Urban JA. Cancers metastatic to the breast. Cancer
1972;29:1691-6.[LinkOut]
- Di Loreto C, Di Lauro V, Puglisi F, Damante G, Fabbro D, Beltrami CA.
Immunocytochemical expression of tissue specific transcription factor-1 in
lung carcinoma. J Clin Pathol 1997;50:30-2.[LinkOut]
- Rhodes A, Jasani B, Balaton AJ, Barnes DM, Miller KD. Frequency of
oestrogen and progesterone receptor positivity by immunohistochemical
analysis in 7016 breast carcinomas: correlation with patient age, assay
sensitivity, threshold value, and mammographic screening. J Clin Pathol
2000;53:688-96.[LinkOut]
- Nadj i M, Gome z -Fernande z C, Ganje i-Az ar P, Mora l es AR .
Immunohistochemistry of estrogen and progesterone receptors
reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol
2005;123:21-7.[LinkOut]
- Dennis JL, Hvidsten TR, Wit EC, Komorowski J, Bell AK, Downie I, et al.
Markers of adenocarcinoma characteristic of the site of origin: development
of a diagnostic algorithm. Clin Cancer Res 2005;11:3766-72.[LinkOut]
- Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an
immunohistologic validation survey for sensitivity and specificity. Am J
Clin Pathol 2007;127:103-13.[LinkOut]
- Takeda Y, Tsuta K, Shibuki Y, Hoshino T, Tochigi N, Maeshima AM,
et al. Analysis of expression patterns of breast cancer-specific markers
(mammaglobin and gross cystic disease fluid protein 15) in lung and
pleural tumors. Arch Pathol Lab Med 2008;132:239-43.[LinkOut]
- Yang M, Nonaka D. A study of immunohistochemical differential
expression in pulmonary and mammary carcinomas. Mod Pathol
2010;23:654-61.[LinkOut]
Cite this article as: Ji FF, Gao P, Wang JG, Zhao J, Zhao P. Contralateral
breast metastasis from pulmonary adenocarcinoma: two cases report
and literature review. J Thorac Dis 2012;4(4):384-389. doi: 10.3978/
j.issn.2072-1439.2012.02.03
|