
Airway pharmacology: treatment options and algorithms to treat patients with chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) as a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases (1). This review will deal with the pharmacology of the treatable component, acknowledging that there are several highly effective non-pharmacological treatments that have an important place in COPD management (1).
Pharmacological treatment of COPD aims to both reduce the burden of COPD and to prevent future risk, especially of exacerbations, hospitalizations, further decline especially of lung function and ultimately mortality (1). Reducing the burden primarily signifies reducing symptoms, improving quality of life, and increasing exercise capacity.
Targets for current pharmacological treatments include especially smooth muscle contraction, inflammation, mucus production, alpha-1-antitrypsin deficiency, and infection. There are basically 5 categories of medications commonly used in the treatment of COPD. Bronchodilators are the mainstay, and as secondary choice agents methylxanthines. Anti-inflammatory therapy can be administered as corticosteroids, inhaled or systemic, as phosphodiesterase (PDE) inhibitor, and probably by long-term antibiotics such as azithromycin. Problems from mucus production are addressed by mucolytics, and in many countries alpha-1-antitrypsin augmentation therapy is available. The treatment of bacterial infection and/or colonization can be attempted with antibiotics; there is a dire need for effective anti-viral agents for the common viruses causing exacerbations of COPD. Smoking cessation, and nicotine replacement or other pharmaceutical aids, and vaccines are outside the scope of this review.
We will first briefly describe the basic pharmacology of the different classes of agents, followed by the effects they exert in patients with COPD, and the side effects.
Since clinicians need to choose medications for their individual patients, algorithms for how to choose and change medication are increasingly being presented with more elements of personalized medicine. We will describe the concept of treatable traits, and discuss in more detail the 2019 GOLD algorithm. All medications are enumerated in Table 1.
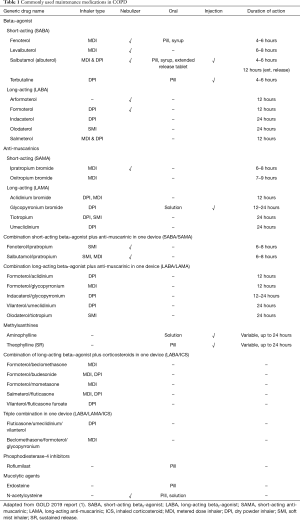
Full table
Pharmacological mechanisms
Bronchodilators
Bronchodilators are named because of their role in increasing the diameter of the airways, especially by relaxing smooth muscle contraction.
Bronchodilators are the mainstay of COPD treatment, and it is therefore remarkable that less than 10 years ago, many texts including GOLD still defined COPD as a disease with irreversible airways obstruction, to which it seems counterintuitive to administer a bronchodilator. The current definition of persistent or chronic obstruction refers to the notion that under no circumstance does the obstruction reverse fully to normal. In fact, some patients have large reactions to one or more of the bronchodilators, whereas others have little or no reaction which also varies over time. In other words, the contribution of smooth muscle contraction varies per person and over time, as does the contribution of increased cholinergic tone.
Anti-muscarinics
Inhaled anti-muscarinics are among the oldest therapies for obstructive airways diseases; for instance, they were long ago added to cigarettes as abstract from datura stramonium. Anti-muscarinics act by blocking the muscarinic receptor of which humans have at least 5 sub-types, M1–M5. Stimulation of M3 receptors on smooth muscle and M1 results in bronchoconstriction, whereas the M2 receptor acts protective against such an effect. Preferably therefore M1 and M3 should be antagonized and not M2. Fully sparing M2 has proven to be difficult so far and current formulations, especially the long-acting ones, have much longer binding to M3 than to M2 thereby in balance working well (1,2).
Beta2-agonists
Beta2-agonists stimulate beta2-receptors on smooth muscle, thereby causing cAMP release and subsequently smooth muscle relaxation. There are many short-acting inhaled beta2-agonists available of which salbutamol (named albuterol in some countries) is the archetype. These short-acting beta2-agonists typically work quite fast (5–15 minutes), slightly faster than anti-muscarinics. The duration of action is 4–6 hours making repeated administration for maintenance necessary. Long-acting inhaled beta2-agonists work 12–24 hours but differ in their onset of action: e.g., formoterol, indacaterol after 5–15 minutes whereas the oldest available salmeterol 30 minutes. There are also differences in being full or partial beta2-agonists, but whether that is clinically relevant has not been proven.
Methylxanthines
Methylxanthines have a modest position in the treatment of COPD according to many experts. Nevertheless, the use of this drug prevails and there are considerable differences between countries in the frequency of their usage. Theophylline, a very old drug but still the central exponent of the class, is generally thought to be a non-selective PDE inhibitor, giving rise to limited bronchodilation and many unwanted effects. Good data on onset and duration of action are lacking.
Anti-inflammatory medication
Corticosteroids
Corticosteroids are well known for a broad range of anti-inflammatory effects, as well as side effects. Inflammation, and its importance in COPD, is not very well understood but is heterogeneous between patients and perhaps within patients over time. The nature and site of the inflammation determines whether (inhaled) anti-inflammatory agents work. Frequently, neutrophilic inflammation is the most prominent and this tends to be minimally responsive to steroids in vitro and in vivo. In some patients with COPD, there is additionally an eosinophilic component (3) which is usually more responsive to steroids. Pauci-inflammatory phenotypes also exist (4).
Synergistic effects of corticosteroids with beta2-agonists and with anti-muscarinics have been suggested from in vitro work, but have not been demonstrated in humans with COPD. They do act additively, vide infra.
PDE-4 inhibitor
PDE-4 inhibition acts intracellularly by metabolizing a cyclic AMP-enzyme in inflammatory cells such as macrophages, eosinophils, and neutrophils. This increases cAMP and activates protein kinase A. This reduces inflammation and as currently thought only has indirect bronchodilatory effects in COPD. The effects take several weeks to kick in.
Antibiotics
Next to bactericidal or bacteriostatic mechanisms, some antibiotics especially from the macrolide group also have anti-inflammatory and perhaps antiviral effects. The best evidence is for azithromycin which has several effects on macrophage function and on the release of many pro-inflammatory cytokines. Airway epithelial cell function improves and mucus production can be reduced.
Mucolytics
The group of mucolytics (erdosteine, carbocysteine, n-acetylcysteine) has heterogeneous targets, all trying to improve mucus rheology. They break the disulfide bonds of mucus thereby reducing its viscosity, and some are also radical scavengers (erdosteine, N-acetylcysteine). Successful reduction of mucus production has not yet been convincingly demonstrated.
Alpha-1-antitrypsin replacement
Alpha-1-antitrypsin deficiency is a well-known but rare recessive disorder leading to insufficient inhibition of alpha-1-proteinases (especially elastase) from activated neutrophils throughout the body. The effects and severity are dependent on the exact mutations, and frequently involve the lung (emphysema) and or the liver. Replacement, only in the specific genetically deficient patients, can be done by weekly intravenous injections of human blood derived protein.
Effects and adverse effects of medication: stable state
Anti-muscarinics
The most frequently used short-acting inhaled anti-muscarinic, ipratropium, typically works after 30–45 minutes for 6–8 hours. The long-acting anti-muscarinics work longer (12–24 hours), and typically also exert more bronchodilation than the short-acting variants. Where available and affordable, the long-acting formulations are to be preferred.
Long-acting anti-muscarinics improve quality of life and symptoms, improve exercise tolerance and the effects of rehabilitation, and reduce exacerbation frequency and hospitalization rates (1), probably all by relaxing the smooth muscles, thereby stenting the airways and increasing the patency. Additionally, there is ongoing debate on possible anti-inflammatory effects of muscarinic blockade, stemming from the abundance of muscarinic receptors and acetyl transferase on many inflammatory cells and based on interesting animal work documenting anti-inflammatory and anti-remodeling effects (5,6).
The most important side effect is dry mouth, which is seldom severe enough to withdraw treatment. There has been discussion regarding a potential adverse effect on cardiac deaths especially with tiotropium in the Respimat formulation but this was refuted in a large subsequent trial (7). There is less long-term evidence with the other formulations.
Beta2-agonists
Beta2-agonists produce bronchodilation resulting in improved FEV1 and to a lesser degree FVC. As with all bronchodilators this as a rule also results in decrease in hyperinflation when present in the individual. Long-acting beta2-agonists typically are more effective at (sustained) bronchodilation than short-acting formulations and should therefore be preferred when available and affordable. The argument of fast onset of action applies also to some of the long-acting formulations and is not a reason to prefer the short-acting.
Long-acting beta2-agonists improve quality of life and symptoms, improve exercise capacity, reduce exacerbation and hospitalization rates. The reduction in exacerbation rate, has in two large trials been suggested to be slightly lower compared to long-acting anti-muscarinics (8,9).
Adverse effects with beta2-agonists are more frequent than with anti-muscarinics and are related to stimulating (other) systemic beta-receptors especially in the heart. Use causes a slight increase in heartbeat, with sometimes arrhythmias that can be bothersome and prohibitive in some people. Some people experience disturbing tremors. Both these adverse effects have a marked tachyphylaxis within days, which is larger than the tachyphylaxis of the bronchoprotective effect. The bronchodilatory effect shows little or no discernible tachyphylaxis. The discussion on potential increased death rates in asthmatics using (high doses of) beta2-agonists has not been extended into COPD which by itself is remarkable.
Combination inhaled therapy: two bronchodilators
The combination of two types of bronchodilators, short-acting or long-acting has consistently been shown to be more effective in improving lung function and symptoms than either component alone. Several, though not all studies that have focused on exacerbations found that the combination of long-acting bronchodilators yields greater reduction in exacerbation rates than the components alone (1,10,11), but it is important to understand that the definitions of exacerbations have varied considerably from mild to severe and combinations thereof. Some studies found reductions in exacerbations based largely on the milder variants of the exacerbations (10). One study actually found no additional reduction when adding olodaterol to tiotropium (12).
Inhaled corticosteroids (ICS)
There is very little evidence of a favorable risk benefit ratio of ICS without concomitant long-acting bronchodilation in pure COPD (1). The effect on decline in lung function of approximately 7 mL/year is too small to be relevant (13); no pharmacological treatment to date has been shown to reduce decline in COPD, as opposed to especially smoking cessation. Whether the effect is better in patients with eosinophilia has not yet been investigated, whereas it has been established for patients with eosinophilia on combined drugs regimens containing ICS (vide infra).
Adverse effects of ICS are discussed under the heading triple combination therapy.
Combination inhaled therapy: long-acting bronchodilator with corticosteroid
As stated above, there is no place for ICS mono-therapy in COPD in general, and there has been quite some debate on the value of ICS added to one or two long-acting bronchodilators. In balance there is now sufficient evidence to state that the combination of inhaled corticosteroid with long-acting beta2-agonist has greater improvements in quality of life, lung function, and exacerbation rates in patients with GOLD lung function grade II–IV, than either components alone (1,13,14). The reduction in number of exacerbations is between 17–24% (14) and seems to be dependent on eosinophil number in the blood; for more detail see further down in section eosinophilia.
Combination inhaled therapy: triple therapy
There are now at least ten randomized controlled trials (RCTs) of triple therapy, which differed considerably in design, ICS washout, length of run-in, and in- and exclusion criteria. Some of these studies were with the triple therapy in different devices and/or not with the same drugs in the comparison (other long-acting beta-agonists for instance). In balance, triple was proven to be superior to one, or two long-acting bronchodilators combined, and to the combination of inhaled corticosteroid and long-acting beta2-agonist in terms of lung function, symptoms, quality of life, and exacerbation frequency (15) and therefore GOLD now states the evidence as level A (1). The largest study by Lipson et al. in over 10,000 patients assessed the same components in the same dosages in the same device in three arms: fluticasone furoate plus vilanterol plus umeclidinium to the first two drugs, and to the last two, all administered double blind in one single dry powder inhaler. It demonstrated a 15% and 25% reduction in exacerbation rate with the triple compared to the named dual combinations, respectively. In the secondary end-points, this was accompanied by a significant reduction in hospitalizations and mortality (16). For treatment algorithms and personalized treatment, vide infra.
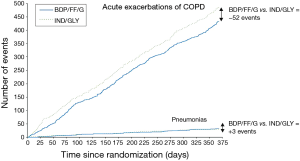
The use of ICS is associated with increased risk of pneumonia (1). In the above-mentioned study (16), the hazard ratio for pneumonia was 1.53. This increased rate of pneumonia makes sense from a pharmacological (immunosuppressive) point of view, but has been more clearly documented for some ICS than for others. There are however no direct head to head comparisons to advise preference of drug. More importantly, and perhaps more than is currently done, this negative effect of steroids eliciting a slight rise in pneumonia should be balanced against the much greater reduction in exacerbations see Figure 1 (17), hospitalizations, and perhaps mortality (16,18). Other adverse effects with ICS are manifold including classically dysphonia, candidiasis and skin bruising.
Systemic corticosteroids
There is no randomized controlled evidence of favorable effects of maintenance dosing systemic steroids in COPD (1), whereas their side effects are numerous. The systemic side effects are considerably more severe than those of ICS.
PDE-4 inhibitor
To date there is only one PDE-4 inhibitor, roflumilast, which is administered orally once daily. It leads to a reduction in exacerbation frequency in patients uncontrolled on combination inhaled medication with complaints of chronic cough and sputum production (chronic bronchitis) with an FEV1 below 50% predicted, and a history of exacerbations (1). The effect seems to be larger in patients with more exacerbations, hospitalizations, and eosinophilia (19).
Oral PDE-4 inhibition with roflumilast leads to several adverse effects including diarrhea, nausea, sleep disturbance and headache which reduces willingness to take or continue the medication. Many patients also experience weight loss, especially in the first few weeks of treatment.
Maintenance antibiotics
Azithromycin and in one study erythromycin have been shown to reduce exacerbation rates in patients with COPD and frequent exacerbations on top of usual care (20,21). The effects are not only attributed to their antibiotic affect but also to a putative anti-inflammatory effect, and seem to be larger in ex-smokers than in current smokers (22).
Of other antibiotics, there is no compelling data supporting maintenance dosing in COPD.
A major discussion surrounding the maintenance use of antibiotics is the development of resistance. With 1-year use of azithromycin, this has not been consistently demonstrated but with more sophisticated molecular techniques, it clearly does exist. The exact clinical relevance has not been fully elucidated. Important other side effects include prolongation of the QTC interval, and hearing loss.
Mucolytics
As a group, mucolytics have been evaluated in meta-analyses to have modest reductions in exacerbation frequency and partially also improve quality of life (1). Many of the studies have been performed in patients with chronic bronchitis, without or with (COPD) obstruction. Perhaps most widely studied in RCTs of sufficient length is N-acetylcysteine but with contradictory results.
Alpha-1-antitrypsin replacement
The effects of alpha-1-antitrypsin replacement have not been as thoroughly documented as should be performed. Especially, there is still no well-designed RCT of sufficient length showing a decreased decline in spirometric parameters. Several cohort studies in balance however, do suggest such an effect (1), as do studies of CT-derived lung density parameters (23).
The side effects of replacement are those of infusions of proteins derived from other people’s blood, including transfusion and allergic reactions and theoretically a risk of transmission of infections.
Exacerbations
Systemic corticosteroids
Whereas the role of systemic corticosteroids in stable state COPD is limited as described above, its role during exacerbations is much more established, improving recovery time and risk of relapse (1). Nevertheless, its use, versus the use of antibiotics differs by country and even by physician. Comparisons of dosage and length have not been carried out to any sufficient degree, but when tested, lower and shorter has been suggested to be as good as higher and longer (24,25).
Differences between formulations, delivery modes, and devices
There is an overwhelming paucity of direct comparisons of drugs, devices and even dosages. Some drugs or their combinations are available in dry powder and in metered dose aerosols but have not been compared. We believe at current there is no reason to prefer one over the other when inhalation technique suffices in a patient; in elderly patients with less coordination, metered dose aerosol with chamber is probably the best delivery mode. Many patients are happy with nebulizations but proper RCTs are scarce and greater effect has not been clearly shown yet (26). Moreover, in many countries there is no nebulized form of long-acting drugs or only beta2-agonists available. It is intuitive to expect greater ease of use and perhaps adherence when combinations of drugs can be administered in a single device, and once daily versus twice daily, but the added value of this has not been proven.
Personalized treatment versus one size fits all
Algorithms to base treatment decisions on have traditionally been viewed from the group level, i.e., in this case as applicable to the whole group of patients with COPD. This led to a one size fits all treatment. This has many disadvantages amongst others that it encompasses that COPD is one homogenous disease, and that all patients within a group also react similarly. Both are clearly wrong and increasingly treatment choices are individualized and moreover subsequently taken in shared decision with the patient.
Agusti and others have recently made a major contribution to the individualization of treatment advices by introducing in several manuscripts the idea treatable traits in obstructive airways diseases (27). The idea actually encompasses two important related changes: first is not to get caught up by a disease label (COPD, or asthma, or overlap syndromes), and second to characterize individual patients by traits that have real treatment consequences, be it to start or stop a treatment. Actually, in its last treatment algorithm for follow-up pharmacological treatment, GOLD employs 7 treatable traits: dyspnea, exacerbations, pneumonia, chronic bronchitis, smoking, low FEV1, and eosinophilia. The role of eosinophilia is further discussed below.
Eosinophilia
Perhaps the most recent practical contribution to personalized treatment of COPD has been the use of (blood) eosinophilia. Although long seen as typical trait in asthma, blood eosinophilia occurs in a considerable number of patients with COPD. Importantly, it is associated with reduction in exacerbation frequency in response to ICS (3). Although not tested prospectively in an algorithm, data derived from large RCTs suggest a continuum of response, with blood eosinophilia below 100 suggesting little or no benefit from adding ICS to long-acting beta2-agonists or dual long-acting bronchodilators; values above 300 are associated with high response rates (28,29). These levels are now reflected in the GOLD treatment algorithm (1). Levels between 100–300 need most careful consideration in partnership with the patient, weighing the burden, predicted effects, and side effects (30).
At least two studies have also suggested it to be safe to limit the administration of systemic corticosteroids during exacerbations to patients with blood eosinophilia, both outside (31) and inside the hospital (32).
Discussion
With increasing numbers of relevant traits, it will be increasingly difficult to use them combined in one algorithm (e.g., what about a smoking person, with eosinophilia and frequent exacerbations, etc.). Algorithms do not yet incorporate any endotyping such as gene expression or microRNAs, but adding this will hopefully help to further personalize treatment choices, probably with the help of computerized algorithms based on big data network analyses (33). The ongoing challenge for clinical practice is how to implement personalized medicine for people with COPD, by combining advances in pharmacological therapy with clinical and molecular treatable traits that drive response to therapy.
Acknowledgments
None.
Footnote
Conflicts of Interest: HA Kerstjens reports research grants from GSK, Novartis, and Boehringer, and fees for consultancies in advisory boards from other from GSK, Novartis, and Boehringer, all paid to his institution. JW Upham has served on Advisory Boards for GSK, AZ, Novartis and Boehringer Ingelheim and has received speaker fees from GSK, AZ, Novartis and Boehringer Ingelheim. IA Yang has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2019 report. 2019 19-08-2019. Available online: http://goldcopd.org/
- Melani AS. Long-acting muscarinic antagonists. Expert Rev Clin Pharmacol 2015;8:479-501. [Crossref] [PubMed]
- Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015;3:435-42. [Crossref] [PubMed]
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011;184:662-71. [Crossref] [PubMed]
- Gosens R, Zaagsma J, Meurs H, et al. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 2006;7:73. [Crossref] [PubMed]
- Pera T, Zuidhof A, Valadas J, et al. Tiotropium inhibits pulmonary inflammation and remodelling in a guinea pig model of COPD. Eur Respir J 2011;38:789-96. [Crossref] [PubMed]
- Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013;369:1491-501. [Crossref] [PubMed]
- Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093-103. [Crossref] [PubMed]
- Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet 2013;1:524-33. [PubMed]
- Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbatons with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parellel-group study. Lancet Respir Med 2013;1:199-209. [Crossref] [PubMed]
- Pavord ID, Lettis S, Locantore N, et al. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax 2016;71:118-25. [Crossref] [PubMed]
- Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6:337-44. [Crossref] [PubMed]
- Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012.CD002991. [PubMed]
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012.CD006829. [PubMed]
- Zheng Y, Zhu J, Liu Y, et al. Triple therapy in the management of chronic obstructive pulmonary disease: systematic review and meta-analysis. BMJ 2018;363:k4388. [Crossref] [PubMed]
- Lipson DA, Barnhart F, Brealey N, et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N Engl J Med 2018;378:1671-80. [Crossref] [PubMed]
- Vanfleteren L, Fabbri LM, Papi A, et al. Triple therapy (ICS/LABA/LAMA) in COPD: time for a reappraisal. Int J Chron Obstruct Pulmon Dis 2018;13:3971-81. [Crossref] [PubMed]
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89. [Crossref] [PubMed]
- Martinez FJ, Rabe KF, Calverley PMA, et al. Determinants of Response to Roflumilast in Severe Chronic Obstructive Pulmonary Disease. Pooled Analysis of Two Randomized Trials. Am J Respir Crit Care Med 2018;198:1268-78. [Crossref] [PubMed]
- Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689-98. [Crossref] [PubMed]
- Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2014;2:361-8. [Crossref] [PubMed]
- Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med 2014;189:1503-8. [Crossref] [PubMed]
- Chapman KR, Burdon JG, Piitulainen E, et al. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet 2015;386:360-8. [Crossref] [PubMed]
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013;309:2223-31. [Crossref] [PubMed]
- Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-7. [Crossref] [PubMed]
- van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev 2016.CD011826. [PubMed]
- Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016;47:410-9. [Crossref] [PubMed]
- Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018;6:117-26. [Crossref] [PubMed]
- Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med 2019;7:745-56. [Crossref] [PubMed]
- van den Berge M, Kerstjens HA. Blood eosinophils as a continuous variable in the treatment of COPD: impact on the guidelines. Lancet Respir Med 2019;7:722-3. [Crossref] [PubMed]
- Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012;186:48-55. [Crossref] [PubMed]
- Sivapalan P, Lapperre TS, Janner J, et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicentre, randomised, controlled, open-label, non-inferiority trial. Lancet Respir Med 2019;7:699-709. [Crossref] [PubMed]
- Agusti A, Anto JM, Auffray C, et al. Personalized respiratory medicine: exploring the horizon, addressing the issues. Summary of a BRN-AJRCCM workshop held in Barcelona on June 12, 2014. Am J Respir Crit Care Med 2015;191:391-401. [Crossref] [PubMed]


